Development of Itaconate Polymers Microparticles for Intracellular Regulation of Pro-Inflammatory Macrophage Activation
- PMID: 40183748
- PMCID: PMC12083439
- DOI: 10.1002/adhm.202405257
Development of Itaconate Polymers Microparticles for Intracellular Regulation of Pro-Inflammatory Macrophage Activation
Abstract
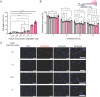
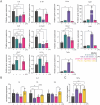
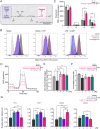
Itaconate (IA) is an endogenous metabolite and a potent regulator of the innate immune system. It's use in immunomodulatory therapies has faced limitations due to challenges in controlled delivery and requirements of high extracellular concentrations for internalization of the highly polar small molecule to achieve its intracellular therapeutic activity. Microparticle (MP)-based delivery strategies are a promising approach for intracellular delivery of small molecule metabolites through macrophage phagocytosis and subsequent intracellular polymer degradation-based delivery. Toward the goal of intracellular delivery of IA, degradable polyester polymer- (poly(dodecyl itaconate)) based IA polymer microparticles (IA-MPs) are generated using an emulsion method, forming micron-scale (≈1.5 µm) degradable microspheres. IA-MPs are characterized with respect to their material properties and IA release kinetics to inform particle fabrication. Treatment of murine bone marrow-derived macrophages with an optimized particle concentration of 0.1 mg million-1 cells enables phagocytosis-mediated internalization and low levels of cytotoxicity. Flow cytometry demonstrates IA-MP-specific regulation of IA-sensitive inflammatory targets. Metabolic analyses demonstrate that IA-MP internalization inhibits oxidative metabolism and induced glycolytic reliance, consistent with the established mechanism of IA-associated inhibition of succinate dehydrogenase. This development of IA-based polymer microparticles provides a basis for additional innovative metabolite-based microparticle drug delivery systems for the treatment of inflammatory disease.
Keywords: biomaterials; immunometabolism; inflammation; microparticles; polyester.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
K.W., N.C., and L.D.H. hold intellectual property rights related to poly(dodecyl itaconate) degradable polyester particles for intracellular itaconate delivery.
Figures


Update of
-
Development of itaconate polymer microparticles for intracellular regulation of pro-inflammatory macrophage activation.bioRxiv [Preprint]. 2025 Feb 5:2025.01.30.635692. doi: 10.1101/2025.01.30.635692. bioRxiv. 2025. Update in: Adv Healthc Mater. 2025 May;14(13):e2405257. doi: 10.1002/adhm.202405257. PMID: 39974988 Free PMC article. Updated. Preprint.
References
-
- Fujiwara N., Kobayashi K., Curr. Drug Target: Inflammation Allergy 2005, 4, 281. - PubMed
MeSH terms
Substances
Grants and funding
- PJT - 191692/Canadian Institutes for Health Research Project
- RGPIN-2022-03666/Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery
- R01AI155907/National Institute of Allergy and Infectious Diseases
- 01AR078343/AR/NIAMS NIH HHS/United States
- R01 AI155907/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

