Seeding-competent early tau multimers are associated with cell type-specific transcriptional signatures
- PMID: 40183825
- PMCID: PMC11971191
- DOI: 10.1007/s00401-025-02869-4
Seeding-competent early tau multimers are associated with cell type-specific transcriptional signatures
Abstract
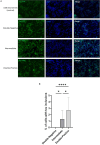
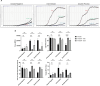
The initial molecular alterations of Alzheimer's disease (AD) are unknown. Established AD is characterized by profound structural and transcriptional alterations in the human brain, with the hallmark neuropathological features being beta-amyloid (Aβ) accumulation in senile plaques and hyperphosphorylated fibrillar tau in neurofibrillary tangles (NFTs). Previous evidence indicates that tau multimerization into small aggregates is one of the earliest molecular alterations, anticipating the accumulation of hyperphosphorylated tau in NFTs. In this study, we investigated the seeding capacity of these early small tau multimers and the transcriptional changes associated with them, aiming to unveil early pathogenic processes in AD-type tau pathology. Early tau multimers visualized with tau proximity ligation assay (tau-PLA) in the post-mortem temporal cortex demonstrated high seeding activity detected by real-time quaking-induced conversion (RT-QuIC) assay and induction of aggregates in a tau biosensor cell line. Using single-nucleus transcriptomics, we showed that brain tissue harboring seeding-competent early tau multimers, but without significant NFT pathology, is associated with substantial gene expression alterations across diverse cell types when compared to control tissue lacking either multimers or NFTs. Differentially expressed genes, such as APP, MAPT, and PSEN1, exhibited significant enrichment of AD heritability in up-regulated genes within excitatory neurons, astrocytes, and oligodendrocytes. Pseudotime analysis exposed a positive correlation between the progression of tau pathology and the expression of genes marking reactive astrocytes. In summary, our results support the hypothesis that seeding-competent tau multimerization may initiate AD-type tau pathology cascades before the accumulation of tau in NFTs. This research contributes valuable insights into the early molecular events associated with AD, with implications for future diagnostic and therapeutic strategies.
Keywords: Alzheimer's disease; Early pathology; Proximity-ligation assay; Seeding; Single-nucleus RNA sequencing; Tau multimers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures








References
-
- Aran KR, Singh S (2023) Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease—a step towards mitochondria based therapeutic strategies. Aging Health Res 3(4):100169
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

