Evaluation of formulation and immunogenicity of porcine circovirus type 2d (PCV2d) vaccine for needle-free intradermal route injection
- PMID: 40183910
- PMCID: PMC11972937
- DOI: 10.4142/jvs.24323
Evaluation of formulation and immunogenicity of porcine circovirus type 2d (PCV2d) vaccine for needle-free intradermal route injection
Abstract
Importance: Porcine circovirus type 2 (PCV2) is a major pathogen responsible for the porcine circovirus-associated disease, causing significant economic losses in the swine industry worldwide. PCV2d has become the dominant, following a sequential shift from the previously predominant PCV2a and PCV2b genotypes. Although an effective vaccine based on virus-like particles (VLPs) of recombinant PCV2d-based capsid protein has been developed, intramuscular inoculation of the vaccine still induces a wide variety of side effects. In contrast, intradermal vaccination offers benefits, including enhanced immune activation due to the abundance of dendritic cells in the dermal layer, while also reducing side effects.
Objective: Developing a novel vaccine combined with a needle-free inoculation technique is required to elicit protection against PCV2d infection with fewer side effects and higher effectiveness. This study aimed to develop a VLP-based vaccine targeting PCV2d and assess its efficacy when administered intradermally using a needle-free system.
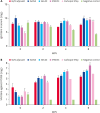
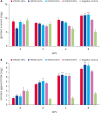
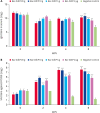
Methods: To optimize the intradermal vaccine formulation, we evaluated humoral immunity and neutralizing activity following intradermal administration of test vaccines prepared with varying adjuvant types, adjuvant ratios, and antigen doses.
Results: IMS1313 adjuvant provided the best induction of total IgG and neutralizing antibody titers. A dose-dependent evaluation indicated that 20 μg of antigen combined with 40% IMS1313 achieved optimal immune responses. Compared to intramuscular injection, intradermal injection using this formulation induced 1.3-fold higher neutralizing antibody titers, demonstrating higher efficacy.
Conclusions and relevance: Intradermal vaccination using a PCV2d VLP-based vaccine improves immunogenicity and cost-effectiveness, providing a promising strategy for controlling PCV2d infections in swine.
Keywords: Porcine circovirus; pcv2d-based vaccine; protective agents; virus-like particle vaccine.
© 2025 The Korean Society of Veterinary Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591–615. - PubMed
-
- Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41(6):624–640. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

