Natural Product Synthesis Enabled by Radical-Polar Crossover Reactions
- PMID: 40183923
- PMCID: PMC7617578
- DOI: 10.1021/acs.joc.5c00306
Natural Product Synthesis Enabled by Radical-Polar Crossover Reactions
Abstract
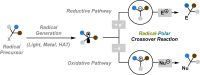
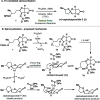
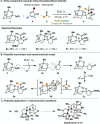
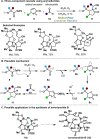
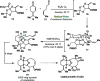
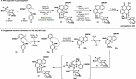
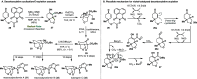
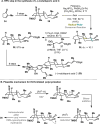
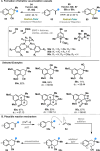
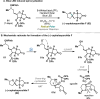
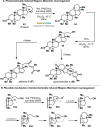
Radical-polar crossover (RPC) chemistry is an emerging field characterized by transformations that involve the coexistence of both radical and ionic species. Since the reactivities of radical and ionic intermediates are orthogonal, applying these two mechanisms in sequence provides significant advantages in the construction of complex molecular architectures. The concept of the RPC approach has become increasingly important in the total synthesis of natural products. This Synopsis presents several examples to showcase recent advancements in this area, including our research on the synthesis of Ganoderma meroterpenoids. In these selected cases, RPC reactions enhance the building of structural complexity and improve overall synthetic efficiency that cannot be achieved by standard synthetic methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Photoredox-Mediated Net-Neutral Radical/Polar Crossover Reactions.Isr J Chem. 2020 Mar;60(3-4):281-293. doi: 10.1002/ijch.201900166. Epub 2020 Feb 18. Isr J Chem. 2020. PMID: 33986554 Free PMC article.
-
New Strategies in the Efficient Total Syntheses of Polycyclic Natural Products.Acc Chem Res. 2020 Nov 17;53(11):2569-2586. doi: 10.1021/acs.accounts.0c00531. Epub 2020 Nov 2. Acc Chem Res. 2020. PMID: 33136373
-
Merging Carbonyl Addition with Photocatalysis.Acc Chem Res. 2022 Apr 19;55(8):1135-1147. doi: 10.1021/acs.accounts.1c00799. Epub 2022 Mar 31. Acc Chem Res. 2022. PMID: 35357118
-
New Methods and Strategies in the Synthesis of Terpenoid Natural Products.Acc Chem Res. 2021 Mar 16;54(6):1347-1359. doi: 10.1021/acs.accounts.0c00809. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596652 Free PMC article. Review.
-
Photo-/Electrocatalytic Difunctionalization of Alkenes Enabled by C-H Radical Functionalization.Chemistry. 2024 Nov 7;30(62):e202402458. doi: 10.1002/chem.202402458. Epub 2024 Oct 16. Chemistry. 2024. PMID: 39126402 Review.
Cited by
-
Total Synthesis of Dactyloquinone A and Spiroetherone A via a Metal-Hydride Hydrogen Atom Transfer (MHAT) Process and a Quinol-Enedione Rearrangement.Angew Chem Int Ed Engl. 2025 Jun 17;64(25):e202505270. doi: 10.1002/anie.202505270. Epub 2025 May 2. Angew Chem Int Ed Engl. 2025. PMID: 40207454 Free PMC article.
References
-
- Sharma S.; Singh J.; Sharma A. Visible Light Assisted Radical-Polar/Polar-Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal. 2021, 363 (13), 3146–3169. 10.1002/adsc.202100205. - DOI
-
- Zhu Z.; Zhang Y.; Li Z.; Shu C. Photoinduced Radical-Polar Crossover Cyclization Reactions. Chem. Catal. 2024, 4 (5), 100945.10.1016/j.checat.2024.100945. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

