Patterns in Prescribing and Dispensing Influenza Antivirals Among Adults With Influenza Presenting to Urgent Care and Emergency Department Settings: VISION Network, 2023-2024
- PMID: 40184184
- PMCID: PMC12353082
- DOI: 10.1093/cid/ciaf178
Patterns in Prescribing and Dispensing Influenza Antivirals Among Adults With Influenza Presenting to Urgent Care and Emergency Department Settings: VISION Network, 2023-2024
Abstract
Background: We describe prescribing and dispensing patterns of influenza antivirals among patients with laboratory-confirmed influenza within US urgent care and emergency department settings.
Methods: A retrospective cross-sectional study was conducted for encounters from 4 large, integrated health systems participating in the VIrtual Sars-cov-2, Influenza, and Other respiratory viruses Network (VISION) of adult patients presenting with acute respiratory illness to urgent care or emergency departments and with positive influenza virus test results during the 2023-2024 influenza season. The analysis was restricted to adult patients at higher risk of influenza complications based on presence of underlying medical conditions, older age, pregnancy, and severe obesity. We calculated proportions and odds of prescribed and dispensed antivirals by demographic and clinical characteristics.
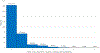
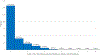
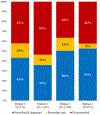
Results: A total of 10 700 patient encounters were eligible for analysis. Among encounters with a positive standard molecular influenza test result (n = 5231), 58% (range across sites: 47%-64%) were prescribed antivirals, with 67% of prescribing occurring on the encounter date. Among those prescribed antivirals (n = 3050), 80% (range across sites: 75%-91%) had them dispensed, with 65% of dispensing occurring on the prescription date. Encounters among persons aged ≥65 years had lower odds of same-day prescribing (.57; 95% CI: .42-.78) and lower odds of same-day dispensing (.58; 95% CI: .36-.94) compared with those aged 18-49 years.
Conclusions: Gaps in antiviral treatment within urgent care and emergency department settings remain for patients at higher risk of influenza complications, notably among older adults. Strategies to improve earlier initiation of antiviral treatment may help reduce the risk of influenza-associated complications.
Keywords: antivirals; dispensing; influenza; oseltamivir; prescribing.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2025.
Conflict of interest statement
Potential conflicts of interest. During the conduct of the study, all Westat-, Kaiser Permanente Southern California Department of Research and Evaluation–, and Kaiser Permanente Northern California Division of Research–affiliated authors reported receiving contractual support from the CDC via payments made to their respective institutions. Additionally, all authors affiliated with Columbia University Irving Medical Center, HealthPartners Institute, Intermountain Healthcare, Kaiser Permanente Center for Health Research, Regenstrief Institute, and University of Colorado Anschutz Medical Campus reported receiving contractual support from the CDC during the conduct of the study, via subcontracts from Westat, Inc, with payments made to their respective institutions. Unrelated to the submitted work, the following disclosures were reported from the past 36 months: S. Y. T. reports contracts from GlaxoSmithKline and Pfizer. N. P. K. reports support from Sanofi, Merck, Pfizer, Seqirus, GlaxoSmithKline, Moderna, AstraZeneca, and Janssen, and is a member of the Board on Population Health and Public Health Practice, National Academies of Science, Engineering, and Medicine, and was on an expert panel sponsored by International Vaccine Institute. S. J. G. reports contracts with the National Institutes of Health (NIH), National Center for Advancing Translational Sciences (NCATS), and NIH National Institute of Mental Health (NIHM). T. C. O. received consulting fees from Regenstrief Institute and support for travel from Patient-Centered Outcomes Research Institute (PCORI) and Regenstrief Institute, and has a current patent: PCT/US2018/047 961. L. S. S. and L. Q. report contracts from GlaxoSmithKline, Moderna, and Dynavax. C. E. M. reports support from NIH, Department of Defense (DOD), PCORI, AstraZeneca, and GlaxoSmithKline and is a member of the American Lung Association of the Minnesota Board, Minnesota Department of Health Long COVID Advisory Committee, and Minnesota Department of Health Asthma Care Advisory Committee. O. Z. reports grant number R01AI168373 with the National Institute of Allergy and Infectious Diseases and support from Pfizer. S. W. B. reports contracts with the University of Utah and Novavax. K. B. J. previously received unrelated research support from Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases. Preliminary Estimated Flu Disease Burden 2023–2024 Flu Season. 2024. [accessed 2024 June 21]; Available from: https://www.cdc.gov/flu-burden/php/data-vis/2023-2024.html
-
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385: 1729–37. - PubMed
-
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clin Infect Dis 2018; 66: 1492–500. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

