Intact HIV DNA decays in children with and without complete viral load suppression
- PMID: 40184428
- PMCID: PMC12002518
- DOI: 10.1371/journal.ppat.1013003
Intact HIV DNA decays in children with and without complete viral load suppression
Abstract
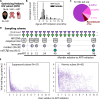
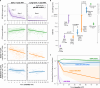
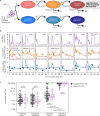
To inform cure in children living with HIV (CWH), we elucidated the dynamics and mechanisms underlying HIV persistence during antiretroviral therapy (ART). In 120 Kenyan CWH who initiated ART between 1-12 months of age, 55 had durable viral load suppression, and 65 experienced ART interruptions. We measured plasma HIV RNA levels, CD4+ T cell count, and levels of intact and defective HIV DNA proviruses via the cross-subtype intact proviral DNA assay (CS-IPDA). By modeling data from the durably suppressed subset, we found that during early ART (year 0-1 on ART), plasma RNA levels decayed rapidly and biphasically and intact and defective HIV DNA decayed with mean 3 and 9 month half-lives, respectively. After viral suppression was achieved (years 1-8 on ART), intact HIV DNA decay slowed to a mean 22 month half-life, whilst defective HIV DNA no longer decayed. In five CWH, we found individual CD4+ TCRβ clones wax and wane, but average kinetics resembled those of defective DNA and CD4 count, suggesting that differential decay of intact HIV DNA arises from selective pressures overlaying normal CD4+ T cell kinetics. Finally, by modeling HIV RNA and DNA in CWH with treatment interruptions, we linked temporary viremia to transient rises in HIV DNA, but long-term intact reservoirs were not strongly influenced, suggesting brief treatment interruptions may not significantly increase HIV reservoirs in children.
Copyright: © 2025 Reeves et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

