Adjuvant Anti-PD-1 Monotherapy Versus Observation for Stage III Acral Melanoma of the Sole: A Multicenter Retrospective Study in Japanese Patients
- PMID: 40184568
- PMCID: PMC12004986
- DOI: 10.1200/GO-24-00644
Adjuvant Anti-PD-1 Monotherapy Versus Observation for Stage III Acral Melanoma of the Sole: A Multicenter Retrospective Study in Japanese Patients
Abstract
Purpose: Adjuvant anti-PD-1 (adj PD-1) antibodies are extensively used to improve survival in patients with resected melanoma. Clinical trials on adj PD-1 antibodies have revealed significant improvements in recurrence-free survival (RFS); however, few of these trials have included patients with acral melanoma (AM).
Methods: Clinical data were retrospectively collected from Japanese patients who underwent resection of stage III sole AM between 2014 and 2021. Survival outcomes, including RFS, distant metastasis-free survival (DMFS), and overall survival (OS), were compared between patients without adjuvant therapy (OBS group) and those receiving adj PD-1 group.
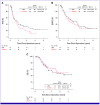
Results: This study included 139 patients (OBS: 79; adj PD-1: 60), with a median follow-up of 2.6 years. The baseline characteristics were comparable, except for age and nodal metastasis. No significant differences in survival were observed between the OBS and adj PD-1 groups (3-year RFS: 36.7% v 27.5%, P = .13; 3-year DMFS: 51.0% v 45.3%, P = .51; 3-year OS: 65.3% v 67.4%, P = .45). Multivariate analysis showed no survival benefit of adj PD-1 (RFS: hazard ratio [HR], 1.25, P = .29; DMFS: HR, 1.03, P = .89; and OS: HR, 0.69, P = .23). Each survival outcome after propensity score matching confirmed no significant difference between the matched OBS group (n = 52) and adj PD-1 group (n = 52; 3-year RFS: 34.3% v 25.9%, P = .22; 3-year DMFS: 45.6% v 46.5%, P = .85; 3-year OS: 60.7% v 68.9%, P = .29).
Conclusion: Adj PD-1 did not improve the prognosis in sole AM. However, further studies are essential to evaluate the efficacy of the adj anti-PD-1 antibody in AM.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Robert C, Grob JJ, Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 - PubMed
-
- Robert C, Carlino MS, McNeil C, et al. : Seven-year follow-up of the phase III KEYNOTE-006 study: Pembrolizumab versus ipilimumab in advanced melanoma. J Clin Oncol 41:3998-4003, 2023 - PubMed
-
- Eggermont AMM, Blank CU, Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

