The FGF13-Caveolin-1 Axis: A Key Player in the Pathogenesis of Doxorubicin- and D-Galactose-Induced Premature Cardiac Aging
- PMID: 40184605
- PMCID: PMC12224973
- DOI: 10.1002/advs.202501055
The FGF13-Caveolin-1 Axis: A Key Player in the Pathogenesis of Doxorubicin- and D-Galactose-Induced Premature Cardiac Aging
Abstract
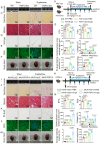
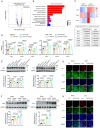
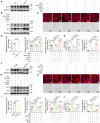
Delaying senescence of cardiomyocytes has garnered widespread attention as a potential target for preventing cardiovascular diseases (CVDs). FGF13 (Fibroblast growth factor 13) has been implicated in various pathophysiological processes. However, its role in premature myocardial aging and cardiomyocyte senescence remains unknown. Adeno-associated virus 9 (AAV9) vectors expressing FGF13 and cardiac-specific Fgf13 knockout (Fgf13KO) mice are utilized to reveal that FGF13 overexpression and deficiency exacerbated and alleviated Doxorubicin/D-galactose-induced myocardial aging characteristics and functional impairment, respectively. Transcriptomics are employed to identify an association between FGF13 and Caveolin-1 (Cav1). Mechanistic studies indicated that FGF13 regulated the Cav1 promoter activity and expression through the p38/MAPK pathway and nuclear translocation of p65, as well as the binding level of PTRF to Cav1 to mediate cardiomyocyte senescence. Furthermore, Cav1 overexpression in murine hearts reversed the alleviatory effects of FGF13 deficiency on the Doxorubicin/D-galactose-induced myocardial aging phenotype and dysfunction. This study has demonstrated that FGF13 regulated the Cav1-p53-p21 axis to augment cardiomyocyte senescence and thereby exacerbated cardiac premature aging and suggests that FGF13 knockdown may be a promising approach to combat CVDs in response to aging and chemotoxicity.
Keywords: cardiac premature aging; cardiomyocyte senescence; caveolin‐1; fibroblast growth factor 13; p53 signaling.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mohamad Kamal N. S., Safuan S., Shamsuddin S., Foroozandeh P., Eur. J. Cell Biol. 2020, 99, 151108. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
