Absence of astrocytic ceruloplasmin reverses the senescence process with aging of learning and memory abilities
- PMID: 40184642
- PMCID: PMC12002888
- DOI: 10.1016/j.redox.2025.103611
Absence of astrocytic ceruloplasmin reverses the senescence process with aging of learning and memory abilities
Abstract
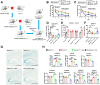
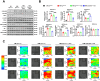
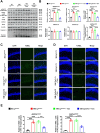
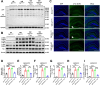
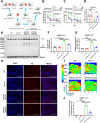
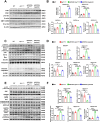
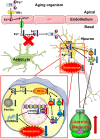
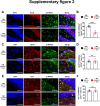
Ceruloplasmin (CP) is a multi-copper ferroxidase mainly synthesized by liver, secreted into the peripheral blood, playing a critical role in regulating the iron homeostasis. In the central nervous system (CNS), the CP expressed by astrocytes plays an important role in the transportation of iron from the blood across the blood-brain barrier (BBB) into the brain. Our previous study showed that conditional knockout of astrocytic CP with Cre-LoxP system (CpGfapcKO) not only improved the learning and memory abilities of elderly mice, but also impaired the learning and memory abilities of young mice. In order to further investigate the effects of CP on learning and memory with aging, we constructed mice model with tamoxifen-induced astrocyte specific knockout of CP, induced CP knockout at 12 months old, and observed the effects on mouse learning and memory at 18 months old. We were delighted to found that ablation of astrocytic CP by tamoxifen at 12 months old could similarly enhance the learning, memory and recognition abilities in 18-month-old mice. Iron deposition in the hippocampus associated with aging was mitigated, leading to a reduction in oxidative stress. The MAPK/JNK pathway exhibited attenuation, while the PI3K/Akt/GSK3 pathway showed enhancement. This combination is expected to result in the reduction of the phosphorylation level of MYC and the elevation of the nuclear translocation of MYC, which might then contribute to reduced cellular senescence. Additionally, the ROS/MAPK/Erk and ROS/MAPK/p38 pathways-dependent cell apoptosis in hippocampus was diminished. The hallmarks of Alzheimer's Disease (AD) were all significantly reduced. Ultimately, the alleviated cellular senescence along with the reduction in AD-related markers, coincided with an improvement in learning, memory, and recognition abilities. These findings further elucidated the role of CP in brain iron metabolism, offering a novel target and strategy for the prevention and treatment of neurodegenerative diseases, such as AD associated with aging.
Keywords: Alzheimer's disease; Astrocytes; Brain iron metabolism; Ceruloplasmin; Recognition; Senescence.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Holmberg C.G., Laurell C.B. Investigations in serum copper; nature of serum copper and its relation to the iron-binding protein in human serum. Acta Chem. Scand. 1947;1:944–950. - PubMed
-
- Holmberg C.G., Laurell C.B. Histaminolytic activity of a copper protein in serum. Nature. 1948;161:236. - PubMed
-
- Patel B.N., David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 1997;272:20185–20190. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

