Efficacy of cognitive rehabilitation in cognition and brain networks: A randomised clinical trial in patients with multiple sclerosis
- PMID: 40184878
- PMCID: PMC11999584
- DOI: 10.1016/j.nicl.2025.103775
Efficacy of cognitive rehabilitation in cognition and brain networks: A randomised clinical trial in patients with multiple sclerosis
Abstract
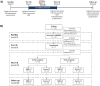
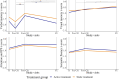
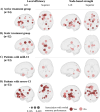
This study evaluated the efficacy of the computerised Guttmann, NeuroPersonalTrainer® (GNPT) cognitive rehabilitation (CR) and characterised the induced changes in cerebral networks in patients with multiple sclerosis (MS). This multicentre, double-blind, randomised clinical trial compared upward intensity training (active treatment) to low-intensity static training (static treatment). Cognition was assessed using the Brief Repeatable battery before and after 12 weeks of training and at 10-months follow-up, and patients were classified as having a mild or severe cognitive impairment (CI). Brain MRI pre- and post-CR were analysed using an advanced tractography algorithm, based on multishell diffusion MRI, to obtain node-based graph metrics (local efficiency and strength) from microscopic fractional anisotropy. Seventy MS patients completed the study (age 48.9 ± 8.8, disease duration 16.8 ± 9.0 years); active treatment: 36, static treatment: 34. Verbal memory improved significantly post-CR in both groups (55 % active; 34 % static treatment), accompanied by increases in local efficiency and strength in multimodal regions. At follow-up, verbal memory declined in both groups but remained above the pre-CR assessment (-25 % and -17 %, respectively). Patients with severe-CI (n = 36) showed improvement only with active treatment, while those with mild-CI (n = 34) improved regardless of intensity treatment. Network changes were more pronounced in patients in active treatment and in those with severe-CI. Quality of life did not change at post-CR, and cognitive improvement was influenced by cognitive reserve (p = 0.011). In MS, GNPT temporarily improves verbal memory and increases network connectivity, reinforcing the CR as a valuable tool for enhancing cognitive skills and promoting neuronal plasticity.
Keywords: Cognition; Cognitive rehabilitation; MRI; Multiple sclerosis; Structural networks.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: EMH, FV, SAA, EA, ERMM, MJA, RSC and MB declare nothing to disclose; ELS holds a grant 100028ID1 AGAUR INVESTIGO22: Programa Investigo. Mecanismo de Recuperación, Transformación y Resiliencia. Funded by the European Union, Next Generation EU. Previously holded a grant from the University of Barcelona predoctoral grant (APIF) and received travel reimbursement from ECTRIMS and Sanofi; AC is supported by the ECTRIMS post-doc fellowship (2022), previously received a UK MS Society PhD studentship (2020), a Guarantors of Brain “Entry” clinical fellowship (2019), and an ECTRIMS-MAGNIMS fellowship (2018). He received travel reimbursement from UK MS society, ECTRIMS, NAIMS; LRP has received in the last three years funding for travel and congress expenses and honoraria for lectures from Biogen, Bristol Myers Squibb, Novartis, Merck, Roche and Teva; SMY has received in the last three years support for congress attendance from Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche and Sandoz. The institution where LRP and SMY work (Hospital Universitari de Bellvitge/ Institut d'Investigació Biomèdica de Bellvitge) has received in the last three years and dedicated exclusively to support the research of the Unit, fees for advisory council, collaborations, donations and advice from Almirall, Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Horizon/Amgen, Janssen, Kern Pharma, Lilly, Merck, Novaxpharm, Novartis, Roche, Sandoz and Sanofi; CRT received research support or compensation for consulting fees, speaker honoraria, support for attending meetings and/or travel, participation on advisory board and research grants for her institution from Biogen Idec, Novartis, Sanofi, Janssen, Bristol, Roche, Almirall, Sandoz and Merck. She holds grants from the ISCIII (PI21/00944; RICORS 21/0002), European grant H2020 (RESTORE – 779316), AGAUR (2021SGR00002); SPR has received travel and congress expenses from Biogen, Novartis and Merck and speaker fees from Biogen and Merck; EM received speaker honoraria from Merck; JM has received personal fees for consulting services and lectures from Novartis, Biogen Idec, Sanofi and Merck-Serono; JSG received compensation in the last 24 months for consulting services and speaking honoraria from BMS, Sanofi, Merck, Janssen, Novartis, and Roche, is scientific director of Revista de Neurología and member of the editorial committee of Multiple Sclerosis Journal. He has received research support from Fondo de Investigación en Salud (PI19/00950 and PI22/00750) from Instituto de Salud Carlos III, Spain; RP receives a grant from the ISCIII (PI19/01634); NSV received funding from the Spanish Government (Instituto de Salud Carlos III, Spain, and Fondo Europeo de Desarrollo Regional [FEDER, FI16/00251] and speaker honoraria from Sanofi, Novartis, Bristol, Myer Squibb, Johnson and Johnson, Biogen-idec, Merck-Serono and Roche; MH received speaker honoraria from Biogen-Idec, Novartis, Merck-Serono, and Sanofi-Genzyme; MS received speaking honoraria from Roche, Biogen, Bial and Horizon Therapeutics and travel reimbursement from Biogen, Sanofi, Merck, Bial and Roche for national and international meetings; AS received compensation for consulting services and speaker honoraria from Merck, Biogen-Idec, Sanofi- Novartis, Roche, Janssen and Horizon Therapeutics; YB received compensation for consulting services and speaker honoraria from Biogen, Novartis, Bristol Myer Squibb Genzyme, Sanofi, Johnson & Johnson, Sandoz and Merck. ES received travel reimbursement from Sanofi, Merck and ECTRIMS; SL received compensation for consulting services and speaker honoraria from Biogen Idec, Novartis, TEVA, Genzyme, Sanofi, Merck and Bristol-Myers Squibb, and holds grants from the Instituto de Salud Carlos III, AGAUR and EME-REEM.
Figures
References
-
- Brissart H., Omorou A.Y., Forthoffer N., Berger E., Moreau T., De Seze J., Morele E., Debouverie M. Memory improvement in multiple sclerosis after an extensive cognitive rehabilitation program in groups with a multicenter double-blind randomized trial. Clin. Rehabil. 2020;34(6):754–763. - PubMed
-
- Chen M.H., Chiaravalloti N.D., DeLuca J. Neurological update: cognitive rehabilitation in multiple sclerosis. J. Neurol. 2021;268(12):4908–4914. - PubMed
-
- Chiaravalloti N.D., DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. - PubMed

