Maternal allostatic load in pregnancy is prospectively associated with child adiposity and metabolic function across infancy and early childhood
- PMID: 40184958
- PMCID: PMC12085287
- DOI: 10.1016/j.psyneuen.2025.107450
Maternal allostatic load in pregnancy is prospectively associated with child adiposity and metabolic function across infancy and early childhood
Abstract
Background: Empirical evidence suggests that the origins of obesity and metabolic dysfunction can be traced to stress-related exposures in prenatal life. The aim of the present study was to examine the prospective association of a composite, multi-system measure of maternal biological stress in pregnancy -- allostatic load (AL) -- with offspring adiposity and insulin resistance across infancy and early childhood.
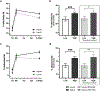
Methods: In N = 55 mother-child dyads, maternal allostatic load was operationalized as a latent variable representing the following components: pre-pregnancy BMI, cortisol, interleukin-6, C-reactive protein, homeostasis model assessment of insulin resistance (HOMA-IR), free fatty acids, and systolic/diastolic blood pressure. Offspring percent total (%FM) and abdominal (%AbFM) fat were quantified with dual-energy X-ray absorptiometry at birth (newborn), 6-mo, and ∼5 yrs age, and HOMA-IR was quantified at ∼5 yrs age. Generalized estimating equation modeling was used to estimate effects of maternal AL on serial (repeated) measures of child adiposity, and linear regression was used to estimate effects on child HOMA-IR. A priori model covariates included maternal race and ethnicity, socioeconomic status, infant feeding practices, child age, and sex.
Results: Maternal AL was positively associated with child %FM and %AbFM before as well as after adjustment for key maternal and offspring covariates (%FM: adjusted β=0.38, p = 0.0074; %AbFM: adjusted β=0.37, p = 0.0013). Maternal AL also was positively associated with child insulin resistance (adjusted β= 0.011, p = 0.0324).
Conclusion: Our findings suggest that exposure to a higher biological stress milieu during prenatal development predisposes towards elevated early life adiposity and insulin resistance in early childhood, a proximate cause of type 2 diabetes and cardiometabolic disease. Collectively, these results provide evidence that a multi-systems approach to quantify early life exposures is useful in prospectively predicting variation in childhood adiposity and metabolic function.
Keywords: Adipose; Allostatic load; Childhood obesity; Fetal programming; Insulin resistance; Stress.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest none
Figures

Similar articles
-
Maternal macronutrient intake at pregnancy and offspring growth trajectory through childhood: a prospective analysis in the Growing Up Today Study 2 cohort.Am J Clin Nutr. 2025 Apr;121(4):843-852. doi: 10.1016/j.ajcnut.2025.01.032. Epub 2025 Feb 1. Am J Clin Nutr. 2025. PMID: 39900248
-
Associations of Abnormal Maternal Glucose Regulation in Pregnancy with Offspring Adiposity, Insulin Resistance, and Adipokine Markers During Childhood and Adolescence.J Pediatr. 2024 Sep;272:114100. doi: 10.1016/j.jpeds.2024.114100. Epub 2024 May 15. J Pediatr. 2024. PMID: 38759779 Free PMC article.
-
Prenatal exposure to per- and polyfluoroalkyl substances and early childhood adiposity and cardiometabolic health in the Healthy Start study.Int J Obes (Lond). 2024 Feb;48(2):276-283. doi: 10.1038/s41366-023-01420-3. Epub 2023 Dec 2. Int J Obes (Lond). 2024. PMID: 38042932 Free PMC article.
-
Insulin for the treatment of women with gestational diabetes.Cochrane Database Syst Rev. 2017 Nov 5;11(11):CD012037. doi: 10.1002/14651858.CD012037.pub2. Cochrane Database Syst Rev. 2017. PMID: 29103210 Free PMC article.
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes.Cochrane Database Syst Rev. 2016 Jun 7;2016(6):CD005542. doi: 10.1002/14651858.CD005542.pub3. Cochrane Database Syst Rev. 2016. PMID: 27272351 Free PMC article.
References
-
- Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats, 2020.
-
- Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics, 1998; 101(3 Pt 2):518–25. - PubMed
-
- Donkor H, Grundt J, Hurum J, et al. 45 Effect of a Multimodal Intervention Program to Prevent Obesity in Early Childhood. Archives of Disease in Childhood, 2012; 97(Suppl 2):A12–A13.
-
- Ghoorah K, Campbell P, Kent A, Maznyczka A, Kunadian V. Obesity and cardiovascular outcomes: a review. Eur Heart J Acute Cardiovasc Care, 2016; 5(1):77–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

