Mutant prion protein enhances NMDA receptor activity, activates PKC, and triggers rapid excitotoxicity in mice
- PMID: 40185484
- PMCID: PMC12077891
- DOI: 10.1172/JCI186432
Mutant prion protein enhances NMDA receptor activity, activates PKC, and triggers rapid excitotoxicity in mice
Abstract
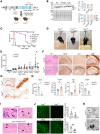
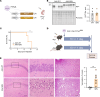
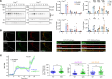
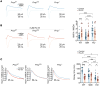
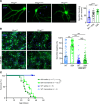
Neuronal hyperexcitability precedes synapse loss in certain neurodegenerative diseases, yet the synaptic membrane interactions and downstream signaling events remain unclear. The disordered amino terminus of the prion protein (PrPC) has been implicated in aberrant signaling in prion and Alzheimer's disease. To disrupt neuronal interactions and signaling linked to the amino terminus, we CRISPR-engineered a knockin mouse expressing mutant PrPC (G92N), generating an N-linked glycosylation site between 2 functional motifs. Mice developed seizures and necrosis of hippocampal pyramidal neurons, similar to prion-infected mice and consistent with excitotoxicity. Phosphoproteomics analysis revealed phosphorylated glutamate receptors and calcium-sensitive kinases, including protein kinase C (PKC). Additionally, 92N-PrPC-expressing neurons showed persistent calcium influx as well as dendritic beading, which was rescued by an N-methyl-d-aspartate receptor (NMDAR) antagonist. Finally, survival of Prnp92N mice was prolonged by blocking active NMDAR channels. We propose that dysregulated PrPC-NMDAR-induced signaling can trigger an excitatory-inhibitory imbalance, spongiform degeneration, and neurotoxicity and that calcium dysregulation is central to PrPC-linked neurodegeneration.
Keywords: Aging; Neurodegeneration; Neuroscience; Prions; Synapses.
Figures


References
MeSH terms
Substances
Grants and funding
- R21 NS105498/NS/NINDS NIH HHS/United States
- R01 AG067049/AG/NIA NIH HHS/United States
- R01 NS076896/NS/NINDS NIH HHS/United States
- T32 OD017863/OD/NIH HHS/United States
- K12 HL141956/HL/NHLBI NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- S10 OD023527/OD/NIH HHS/United States
- RF1 AG067049/AG/NIA NIH HHS/United States
- K99 AG061251/AG/NIA NIH HHS/United States
- RF1 NS121992/NS/NINDS NIH HHS/United States
- T32 AG066596/AG/NIA NIH HHS/United States
- R01 NS069566/NS/NINDS NIH HHS/United States
- R35 AG071734/AG/NIA NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- R37 NS076896/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

