Cancer-associated fibroblasts secrete CSF3 to promote TNBC progression via enhancing PGM2L1-dependent glycolysis reprogramming
- PMID: 40185722
- PMCID: PMC11971334
- DOI: 10.1038/s41419-025-07580-6
Cancer-associated fibroblasts secrete CSF3 to promote TNBC progression via enhancing PGM2L1-dependent glycolysis reprogramming
Abstract
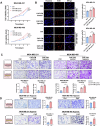
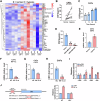
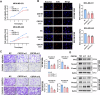
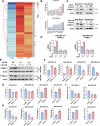
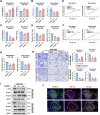
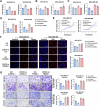
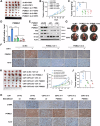
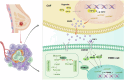
Triple-negative breast cancer (TNBC) is characterized by a pronounced hypoxic tumor microenvironment, with cancer-associated fibroblasts (CAFs) serving as the predominant cellular component and playing crucial roles in regulating tumor progression. However, the mechanism by which CAFs affect the biological behavior of tumor cells in hypoxic environment remain elusive. This study employed a bead-based multiplex immunoassay to analyze a panel of cytokines/chemokines and identified colony stimulating factor 3 (CSF3) as a significantly elevated component in the secretome of hypoxic CAFs. We found that CSF3 promoted the invasive behavior of TNBC cells by activating the downstream signaling pathway of its receptor, CSF3R. RNA sequencing analysis further revealed that phosphoglucomutase 2-like 1 (PGM2L1) is a downstream target of the CSF3/CSF3R signaling, enhancing the glycolysis pathway and providing energy to support the malignant phenotype of breast cancer. In vivo, we further confirmed that CSF3 promotes TNBC progression by targeting PGM2L1. These findings suggest that targeting CSF3/CSF3R may represent a potential therapeutic approach for TNBC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: Human BC tissues and corresponding normal breast tissues were obtained from patients undergoing surgery in Qilu Hospital, Shandong University (Jinan, China). All experimental procedures were approved by the Ethics Committee of Qilu Hospital, Shandong University. The number of ethics approval is KYLL-2022(ZM)-1215. All patients written informed consent for the use of their clinical samples in research. All animal experiments were approved by the Shandong University Animal Care and Use Committee. The diameters of the tumors did not exceed 2 cm, which is the maximum diameter as specified by the committee.
Figures
Similar articles
-
Tumor-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer.Front Immunol. 2019 Apr 12;10:757. doi: 10.3389/fimmu.2019.00757. eCollection 2019. Front Immunol. 2019. PMID: 31031757 Free PMC article.
-
Microenvironmental G protein-coupled estrogen receptor-mediated glutamine metabolic coupling between cancer-associated fibroblasts and triple-negative breast cancer cells governs tumour progression.Clin Transl Med. 2024 Dec;14(12):e70131. doi: 10.1002/ctm2.70131. Clin Transl Med. 2024. PMID: 39690134 Free PMC article.
-
Upregulation of FGF9 and NOVA1 in cancer-associated fibroblasts promotes cell proliferation, invasion and migration of triple negative breast cancer.Drug Dev Res. 2024 May;85(3):e22185. doi: 10.1002/ddr.22185. Drug Dev Res. 2024. PMID: 38657094
-
Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer.Oncotarget. 2016 Dec 13;7(50):82889-82901. doi: 10.18632/oncotarget.12658. Oncotarget. 2016. PMID: 27756881 Free PMC article.
-
Exploring the interplay between triple-negative breast cancer stem cells and tumor microenvironment for effective therapeutic strategies.J Cell Physiol. 2024 Aug;239(8):e31278. doi: 10.1002/jcp.31278. Epub 2024 May 28. J Cell Physiol. 2024. PMID: 38807378 Review.
References
-
- Hu M, Huang L. Strategies targeting tumor immune and stromal microenvironment and their clinical relevance. Adv Drug Deliv Rev. 2022;183:114137. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

