Preclinical concept studies showing advantage of an inhaled anti-CTGF/CCN2 protein for pulmonary fibrosis treatment
- PMID: 40185752
- PMCID: PMC11971276
- DOI: 10.1038/s41467-025-58568-x
Preclinical concept studies showing advantage of an inhaled anti-CTGF/CCN2 protein for pulmonary fibrosis treatment
Abstract
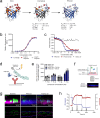
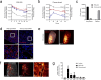
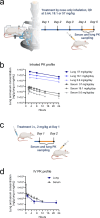
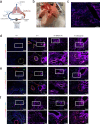
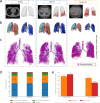
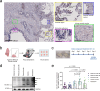
Inhaled therapeutics have high potential for the treatment of chronic respiratory diseases of high unmet medical need, such as idiopathic pulmonary fibrosis (IPF). Preclinical and early clinical evidence show that cellular communication network factor 2 (CCN2), previously called connective tissue growth factor (CTGF), is a promising target for the treatment of IPF. In recent phase 3 clinical trials, however, systemic CCN2 inhibition failed to demonstrate a clinically meaningful benefit. Here, we present the preclinical profile of the inhaled anti-CCN2 Anticalin® protein PRS-220. Our study demonstrates that efficient pulmonary delivery directly translates into superior efficacy in relevant models of pulmonary fibrosis when compared to systemic CCN2 inhibition. Moreover, we present a holistic approach for the preclinical characterization of inhaled PRS-220 from state-of-the art in vitro and in vivo models to novel human ex vivo and in silico models, highlighting the advantage of inhaled drug delivery for treatment of respiratory disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.P., V.N., E.M.H., Cl.W., T.J., S.G., G.M. are co-inventors of an international patent filed by Pieris Pharmaceuticals on the CCN2-targeting Anticalin protein described in this manuscript (WO2022214649A1). V.N., E.M.H, T.J., J.K.G., Co.W., A.K., K.H., A.F., N.S., S.G., D.B., T.M., S.K., M.R., Cl.W., M.F.F., S.A.O., G.M. and M.P. are or were employees of Pieris Pharmaceuticals and may hold stock interests in Pieris Pharmaceuticals. M.J.G, K.R.W, J.B. and K.W.M are employees and/or shareholders of Ebenbuild GmbH and co-inventors on a pending patent application. J.K.B receives research funds from Boehringer Ingelheim. The remaining authors declare no competing interests.
Figures

References
-
- Rau, J. L. The inhalation of drugs: advantages and problems. Res. Care50, 367–382 (2005). - PubMed
-
- West, A. et al. Inhaled pirfenidone solution (AP01) for IPF: a randomised, open-label, dose–response trial. Thorax78, 882–889 (2023). - PubMed
-
- Anselmo, A. C., Gokarn, Y. & Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov.18, 19–40 (2018). - PubMed
-
- Fröhlich, E. & Salar-Behzadi, S. Oral inhalation for delivery of proteins and peptides to the lungs. Eur. J. Pharm. Biopharma.163, 198–211 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

