Impact of inactivated vaccine on transmission and evolution of H9N2 avian influenza virus in chickens
- PMID: 40185759
- PMCID: PMC11971428
- DOI: 10.1038/s41541-025-01115-y
Impact of inactivated vaccine on transmission and evolution of H9N2 avian influenza virus in chickens
Abstract
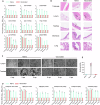
H9N2 avian influenza virus (AIV) is endemic in poultry worldwide and increasingly zoonotic. Despite the long-term widespread use of inactivated vaccines, H9N2 AIVs remain dominant in chicken flocks. We demonstrated that inactivated vaccines did not prevent the replication of H9N2 AIVs in the upper airway of vaccinated chickens. Viral transmission was enhanced during sequential passage in vaccinated chickens, which was attributed to the restricted production of defective interfering particles and the introduction of stable mutations (NP-N417D, M1-V219I, and NS1-R140W) which enhanced viral replication. Notably, the genetic diversity of H9N2 AIVs was greater and included more potential mammal/human-adapted mutations after passage through vaccinated chickens than through naïve chickens, which might facilitate the emergence of mammal-adapted strains. By contrast, vaccines inducing cellular/mucosal immunity in the upper respiratory tract effectively limit H9N2 AIV. These findings highlight the limitations of inactivated vaccines and the need for revised vaccination strategies to control H9N2 AIV.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Zhang, J. et al. Mutational antigenic landscape of prevailing H9N2 influenza virus hemagglutinin spectrum. Cell Rep.42, 113409 (2023). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

