Metagenomic evaluation, antimicrobial activities, and immune stimulation of probiotics from dietary supplements and dairy products
- PMID: 40185827
- PMCID: PMC11971409
- DOI: 10.1038/s41598-025-95664-w
Metagenomic evaluation, antimicrobial activities, and immune stimulation of probiotics from dietary supplements and dairy products
Abstract
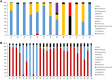
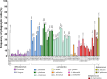
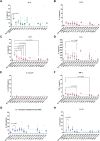
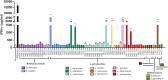
Probiotics are widely marketed as dietary supplements and dairy products for their purported antimicrobial and immunomodulatory activities, often with limited supporting evidence. We identified and isolated probiotics from commercial dietary supplements and dairy products using metagenomics and cultured-based methods. We assessed their anti-bacterial activity against diverse pathogens and investigated their immunomodulatory effects on phagocytes and natural killer (NK) cells. Metagenomic analysis revealed that Lactobacillus and Bifidobacterium were the predominant genera in dietary supplements, while Streptococcus spp. was dominated in dairy products. However, only 37% of the predominant microorganisms identified by metagenomics were accurately listed on product labels. Among 70 representative probiotic strains, 4.3-17.1% probiotic strains demonstrated strong antibacterial-effects against pathogenic bacteria. Notably, specific strains of Bifidobacterium longum and Lactobacillus plantarum exhibited strong antagonistic activity against extended-spectrum beta-lactamase-producing and carbapenem-resistant Escherichia coli. Some strains of Lactobacillus spp. significantly enhanced phagocytic activity in monocytes and increased IFN-γ production in NK cells, while members of Lactobacillus rhamnosus significantly suppressed TNF-α, IL-6, and IL-8 production in lipopolysaccharide-stimulated macrophages. In contrast, Bifidobacterium animalis stimulated the production of anti-inflammatory cytokines. This study highlights discrepancies in probiotic labeling and demonstrates the antimicrobial and immunomodulatory potential of specific probiotic strains, suggesting their utility in enhancing health and wellness.
Keywords: Antimicrobial activity; Dairy products; Immune response; Metagenomic; Probiotics; Supplements.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Gourbeyre, P., Denery, S. & Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol.89, 685–695. 10.1189/jlb.1109753 (2011). - PubMed
-
- Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol.4, 478–485. 10.1038/nri1373 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

