Preference-independent saliency map in the mouse superior colliculus
- PMID: 40185893
- PMCID: PMC11971363
- DOI: 10.1038/s42003-025-08006-x
Preference-independent saliency map in the mouse superior colliculus
Erratum in
-
Author Correction: Preference-independent saliency map in the mouse superior colliculus.Commun Biol. 2025 May 3;8(1):694. doi: 10.1038/s42003-025-08136-2. Commun Biol. 2025. PMID: 40319092 Free PMC article. No abstract available.
Abstract
Detecting salient stimuli in a visual scene is crucial for animal survival, yet how the brain encodes visual saliency remains unclear. Here, using two-photon calcium imaging, we reveal a preference-independent saliency map in the superficial superior colliculus of awake mice. Salient stimuli evoke stronger responses than uniform stimuli in both excitatory and inhibitory neurons, with similar encoding patterns across both cell types. The strongest response occurs when a salient stimulus is centered within the receptive field, with contextual effects extending approximately 40°. Response amplitude scales with saliency strength but remains independent of neurons' orientation or motion direction preferences. Notably, saliency-encoding neurons exhibit weak orientation and direction selectivity, indicating a complementary relationship between saliency and feature maps. Importantly, this preference-independent saliency encoding does not require cortical inputs. These findings provide insights into the neural mechanisms underlying visual saliency detection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

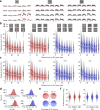


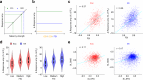


Similar articles
-
Bidirectional encoding of motion contrast in the mouse superior colliculus.Elife. 2018 Jul 2;7:e35261. doi: 10.7554/eLife.35261. Elife. 2018. PMID: 29963987 Free PMC article.
-
Visual receptive field properties of neurons in the superficial superior colliculus of the mouse.J Neurosci. 2010 Dec 8;30(49):16573-84. doi: 10.1523/JNEUROSCI.3305-10.2010. J Neurosci. 2010. PMID: 21147997 Free PMC article.
-
Visual response properties of neurons in the superficial layers of the superior colliculus of awake mouse.J Physiol. 2018 Dec;596(24):6307-6332. doi: 10.1113/JP276964. Epub 2018 Nov 10. J Physiol. 2018. PMID: 30281795 Free PMC article.
-
Effects of Light Isoflurane Anesthesia on Organization of Direction and Orientation Selectivity in the Superficial Layer of the Mouse Superior Colliculus.J Neurosci. 2022 Jan 26;42(4):619-630. doi: 10.1523/JNEUROSCI.1196-21.2021. Epub 2021 Dec 6. J Neurosci. 2022. PMID: 34872926 Free PMC article.
-
Visual Function, Organization, and Development of the Mouse Superior Colliculus.Annu Rev Vis Sci. 2018 Sep 15;4:239-262. doi: 10.1146/annurev-vision-091517-034142. Epub 2018 May 31. Annu Rev Vis Sci. 2018. PMID: 29852095 Review.
References
-
- Li, Z. A new framework for understanding vision from the perspective of the primary visual cortex. Curr. Opin. Neurobiol.58, 1–10 (2019). - PubMed
-
- Treisman, A. M. & Gelade, G. A feature-integration theory of attention. Cogn. Psychol.12, 97–136 (1980). - PubMed
-
- Treisman, A. Preattentive processing in vision. Comput. Vis. Graph. Image Process.31, 156–177 (1985).
-
- Posner, M. I. Orienting of attention. Q. J. Exp. Psychol.32, 3–25 (1980). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

