17β-estradiol status alters NMDAR function and antipsychotic-like activity in female rats
- PMID: 40185905
- PMCID: PMC12339404
- DOI: 10.1038/s41380-025-02996-0
17β-estradiol status alters NMDAR function and antipsychotic-like activity in female rats
Abstract
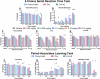
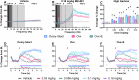
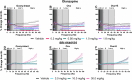
Low 17β-estradiol (E2) in females of reproductive age, and marked E2 decline with menopause, contributes to heightened symptom severity in schizophrenia (i.e. cognitive dysfunction) and diminished response to antipsychotic medications. However, the underlying mechanisms are unknown. N-methyl-D-aspartate receptor (NMDAR) hypofunction contributes to the pathophysiology of schizophrenia, yet impact of E2 depletion on NMDAR function is not well characterized. Quantitative electroencephalography (qEEG), specifically gamma power, is a well-established functional readout of cortical activity that is elevated in patients with schizophrenia and is sensitive to alterations in NMDAR function. Using qEEG and touchscreen cognitive assessments, present studies investigated the effects of E2 on NMDAR function by administering MK-801 (NMDAR antagonist) to ovariectomized rats with or without E2 implants (Ovx+E and Ovx, respectively). Ovx rats were more sensitive to MK-801-induced elevations in gamma power and attentional impairments compared to Ovx+E rats. Further investigation revealed these effects were mediated by reduced synaptic GluN2A expression. Consistent with clinical reports, olanzapine (second-generation antipsychotic) was less effective in mitigating MK-801-induced elevations in gamma power in Ovx rats. Lastly, we examined antipsychotic-like activity of a Group II metabotropic glutamate receptor (mGlu2/3) positive allosteric modulator (PAM), SBI-0646535, as a novel therapeutic in E2-deprived conditions. SBI-0646535 reversed MK-801-induced elevations in gamma power regardless of E2 status. Collectively, these studies established a relationship between E2 deprivation and NMDAR function that is in part GluN2A-dependent, supporting the notion that E2 deprivation increases susceptibility to NMDAR hypofunction. This highlights the need to examine age/hormone-specific factors when considering antipsychotic response and designing novel pharmacotherapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Dr. Cosford has an equity interest in Camino Pharma, LLC, a company that may potentially benefit from the research results.
Figures



Update of
-
17β-estradiol status alters NMDAR function and antipsychotic-like activity in female rats.bioRxiv [Preprint]. 2025 Feb 10:2025.02.10.637465. doi: 10.1101/2025.02.10.637465. bioRxiv. 2025. Update in: Mol Psychiatry. 2025 Sep;30(9):4161-4175. doi: 10.1038/s41380-025-02996-0. PMID: 39990384 Free PMC article. Updated. Preprint.
References
-
- Jang D, Elfenbein HA. Menstrual cycle effects on mental health outcomes: a meta-analysis. Arch Suicide Res. 2019;23:312–32. - PubMed
-
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23:113–22. - PubMed
-
- Hallonquist JD, Seeman MV, Lang M, Rector NA. Variation in symptom severity over the menstrual cycle of schizophrenics. Biol Psychiatry. 1993;33:207–9. - PubMed
-
- Huber TJ, Rollnik J, Wilhelms J, von zur Mühlen A, Emrich HM, Schneider U. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26:27–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

