A Randomized clinical trial evaluating the impact on survival and quality of life of 177Lutetium[Lu]-edotreotide versus everolimus in patients with neuroendocrine tumors of the lung and thymus: the LEVEL study (GETNE T-2217)
- PMID: 40186126
- PMCID: PMC11971812
- DOI: 10.1186/s12885-025-13941-3
A Randomized clinical trial evaluating the impact on survival and quality of life of 177Lutetium[Lu]-edotreotide versus everolimus in patients with neuroendocrine tumors of the lung and thymus: the LEVEL study (GETNE T-2217)
Abstract
Background: Everolimus is the only approved therapy for patients with advanced neuroendocrine tumors (NET) of lung and thymus and new treatment options are urgently needed. Expression of somatostatin receptor 2 (SSTR2) is frequently seen in functional imaging in lung-NETs opening the opportunity to treat SSTR2 positive patients with radioligand therapies (RLT). Retrospective data suggest a potential meaningful benefit of RLT directed to SSTR2 in lung-NET patients.
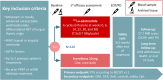
Methods: The LEVEL trial is a randomized, open-label, phase III international trial of 177Lu-edotreotide versus everolimus in patients with progressive, locally advanced or metastatic, and well/moderately differentiated NETs of lung (typical/atypical) or thymic origin. Patients could be treatment-naïve or have progressed (PD) on somatostatin analogues or ≤ 2 additional systemic treatments. Prior RLT or mTOR inhibitors are not permitted. Eligible patients are randomly assigned 3:2 to 6 cycles of 177Lu-edotreotide (total administered activity 7.5 ± 0.7 GBq / cycle) or to oral everolimus 10 mg once daily until PD or unacceptable toxicity. Only patients with positivity in somatostatin receptor imaging will be included. CT or MRI scans are performed every 12 weeks until PD. Blood samples are analyzed at baseline, at 1st tumor assessment, and at PD for pharmacodynamic endpoints. Archival tumor tissue samples will be analyzed for ancillary studies. The primary endpoint is progression-free survival (PFS) according to RECIST v1.1 based on local investigator assessment. Secondary endpoints include overall survival, overall response rate, safety, and quality of life (EORTC QLQ-C30). The expected sample size is 120 patients to demonstrate statistical significant risk reduction of 46.4% (HR = 0.536) in PFS with the experimental treatment using an overall 5% two-sided alpha error with 80% power. An interim PFS analysis was included using the Lan-DeMets with O'Brian-Fleming-like boundaries.
Discussion: The LEVEL trial will investigate if 177Lu-edotreotide has the potential to be incorporated as a standard treatment option for patients with NETs from the lung and Thymus.
Trial registration: EU CT: 2022-502154-13-00 / www.
Clinicaltrials: gov : NCT05918302 (June 23rd, 2023).
Keywords: 177Lu-edotreotide; Everolimus; Lung and Thymus; Neuroendocrine tumors; Targeted Radioligand Therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study is being conducted in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonisation Guidelines for Good Clinical Practice and the local regulations. The study protocol was approved by the IEC of Hospital Universitari Vall D'Hebron on June 19th, 2023 (Ref: 2023/578) and the competent authorities of the European member states concerned (Spain: July 14th, 2023, France: October 10th, 2023, Italy: November 8th, 2023). All patients are providing written informed consent. Consent for publication: Not applicable. Competing interests: RGC has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from: AAA-Novartis, Advanz Pharma, Astellas, Bayer, BMS, Boehringer, Esteve, GSK, Hutchmed, Ipsen, ITM, MSD, PharmaMar, Pierre Fabre, Sanofi, Servier, Takeda.PJF has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from: Astellas, AstraZeneca, Bristol-Myers Squibb (BMS), Esteve, Merck Sharp & Dohme (MSD), Novartis, Nutricia, Pfizer, Rovi, Takeda, Viatris.TW has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from: Novartis, MSD, Esteve, Ipsen, ITM, Terumo, Pierre Fabre, Sanofi, OranoMed, Sirtex.CL has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from: Pierre Fabre, AMGEN, Takeda, Ipsen, AAA.ED has received fees from AAA-Novartis, Janssen and GE.JCR has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from: AMGEN, MSD, AAA.TV has received honoraria for speaker engagements, advisory roles or funding for continuous medical education from from AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Eisai, Elmedix, Ipsen, MyNeoTx, Nordic Pharma, Novartis, Roche, Sirtex, Servier, Takeda. TV is a senior clinical investigator at the Research Foundation-Flanders (FWO), project number 1803723N.The remaining authors declare that they have no competing interests.GV has received a speaker’s fee from Pfizer, MSD, GSK and Pierre Fabrer, has held an advisory role with AstraZeneca and received consultant fees from Reveal Genomics.TAG reports Advisory role or speaker & fee from Lilly, Bayer, Johnson & Johnson, Astellas, Eisai, Roche, Ipsen, MSD and Adacap. Research Grant from Johnson & Johnson and IPSEN.All the other co-authors confirmed that they had no competing interests to be declared.
Figures
References
-
- Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(4):439–51. - PubMed
-
- Korse CM, Taal BG, van Velthuysen MLF, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer Oxf Engl 1990. 2013;49(8):1975–83. - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2015;10(9):1243–60. - PubMed
-
- de Laat JM, Pieterman CR, van den Broek MF, Twisk JW, Hermus AR, Dekkers OM, et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab. 2014;99(9):3325–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous