Accuracy of human epidermal growth factor receptor 2 (HER2) immunohistochemistry scoring by pathologists in breast cancer, including the HER2-low cutoff : HER2 IHC scoring concordance in breast cancer
- PMID: 40186179
- PMCID: PMC11969812
- DOI: 10.1186/s13000-025-01624-3
Accuracy of human epidermal growth factor receptor 2 (HER2) immunohistochemistry scoring by pathologists in breast cancer, including the HER2-low cutoff : HER2 IHC scoring concordance in breast cancer
Abstract
Background: Breast cancer was previously categorized as human epidermal growth factor receptor 2 (HER2)-positive (immunohistochemistry [IHC] 3+, IHC 2+ / in situ hybridization [ISH]-positive) or HER2-negative (IHC 0, IHC 1+, IHC 2+/ISH-). Recent studies of trastuzumab deruxtecan, a HER2-directed antibody-drug conjugate, have explored the spectrum of HER2 expression in tumors categorized as HER2-negative, including HER2-low (IHC 1+, IHC 2+/ISH-) and HER2-ultralow (IHC 0 with membrane staining). Clinical relevance of HER2-low and HER2-ultralow is reinforced by encouraging efficacy findings in these populations.
Objective: To assess HER2-low and HER2-ultralow scoring performance by pathologists, and compare real-world HER2-low scoring with centralized scoring by trained pathologists.
Methods: Formalin-fixed, paraffin-embedded breast cancer samples stained by the VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody (Roche) assay were selected to ensure adequate representation across all HER2 IHC scores (N = 500). Samples were rescored in a central laboratory by three pathologists trained in HER2-low scoring, and a majority consensus generated. Agreement between consensus and historical real-world HER2 scores was assessed by Fleiss' kappa across HER2 scores (IHC 0, 1+, 2+, 3+).
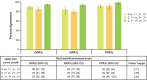
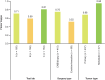
Results: Substantial agreement was observed among central pathologists across HER2 scores (κ = 0.69), for the HER2-low cutoff (IHC 0 vs. IHC 1+, 2+, 3+; κ = 0.79), and the HER2-ultralow cutoff (IHC 0 absent membrane staining vs. IHC 0 with membrane staining, 1+, 2+, 3+; κ = 0.68). Substantial agreement was observed between real-world pathologists and central consensus for the HER2-low cutoff (κ = 0.72).
Conclusions: Pathologists can reproducibly score HER2-low and HER2-ultralow when supported by training. Findings may aid decision-making for patients with breast cancer who are potentially eligible for HER2-directed therapy.
Keywords: Breast cancer; HER2-low; HER2-ultralow; Immunohistochemistry; Scoring.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: AstraZeneca has a governance framework and processes to ensure that commercial sources have appropriate patient consent and ethical approval in place for collection of samples for research purposes, including use by for-profit companies. The AstraZeneca Biobank in the UK is licensed by the Human Tissue Authority (Licence No. 12109) and has National Research Ethics Service Committee (NREC) approval as a Research Tissue Bank (RTB) (REC No 22/NW/0102), which covers the use of the samples for this project. Consent for publication: Not applicable. Competing interests: All authors are employees of AstraZeneca. MV, MS, FJ, TM, A-MB, JW, and CB hold shares in AstraZeneca.
Figures
References
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. - PubMed
-
- Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–95. - PubMed
-
- Wolff AC, Somerfield MR, Dowsett M, Hammond ME, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO–College of American Pathologists guideline update. J Clin Oncol. 2023;41:3867–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

