Fucoidan reduces NET accumulation and alleviates chemotherapy-induced peripheral neuropathy via the gut-blood-DRG axis
- PMID: 40186245
- PMCID: PMC11969723
- DOI: 10.1186/s12974-025-03431-5
Fucoidan reduces NET accumulation and alleviates chemotherapy-induced peripheral neuropathy via the gut-blood-DRG axis
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a serious adverse reaction to chemotherapy with limited treatment options. Research has indicated that neutrophil extracellular traps (NETs) are critical for the pathogenesis of CIPN. LPS/HMGB1 serve as important inducers of NETs. Here, we aimed to target the inhibition of NET formation (NETosis) to alleviate CIPN.
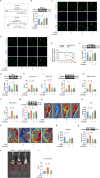
Methods: Oxaliplatin (L-OHP) was used to establish a CIPN model. The mice were pretreated with fucoidan to investigate the therapeutic effect. SR-A1-/- mice were used to examine the role of scavenger receptor A1 (SR-A1) in CIPN. Bone marrow-derived macrophages (BMDMs) isolated from SR-A1-/- mice and WT mice were used to investigate the mechanism by which macrophage phagocytosis of NETs alleviates CIPN.
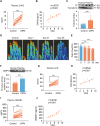
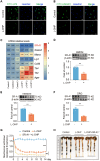
Results: Clinically, we found that the contents of LPS, HMGB1 and NETs in the plasma of CIPN patients were significantly increased and positively correlated with the VAS score. Fucoidan decreased the LPS/HMGB1/NET contents and relieved CIPN in mice. Mechanistically, fucoidan upregulated SR-A1 expression and promoted the phagocytosis of LPS/HMGB1 by BMDMs. Fucoidan also facilitated the engulfment of NETs by BMDMs via the recognition and localization of SR-A1 and HMGB1. The therapeutic effects of fucoidan were abolished by SR-A1 knockout. RNA-seq analysis revealed that fucoidan increased sqstm1 (p62) gene expression. Fucoidan promoted the competitive binding of sqstm1 and Nrf2 to Keap1, increasing Nrf2 nuclear translocation and SR-A1 transcription. Additionally, the sequencing analysis (16 S) of microbial diversity revealed that fucoidan increased the gut microbiota diversity and abundance and increased the Bacteroides/Firmicutes ratio.
Conclusions: Altogether, fucoidan promotes the SR-A1-mediated phagocytosis of LPS/HMGB1/NETs and maintains gut microbial homeostasis, which may provide a potential therapeutic strategy for CIPN.
Keywords: CIPN; Fucoidan; Gut; Macrophage; NETs; SR-A1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was reviewed and approved by the Nanjing First Hospital Institutional Review Committee with ethics number KY20171228-KS-01. All experimental methods were conducted in accordance with relevant guidelines and regulations. The experimental plan involving animals was approved by the Committee of Experimental Animal Ethics of Nanjing Medical University through the “Examination of Ethical Welfare of Experimental Animals” and complies with relevant national welfare ethics provisions for experimental animals. The approval number is IACUC-2011040. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Brown TJ, Sedhom R, Gupta A. Chemotherapy-Induced peripheral neuropathy. JAMA Oncol. 2019;5:750. - PubMed
-
- Battaglini E, Goldstein D, Grimison P, McCullough S, Mendoza-Jones P, Park SB. Chemotherapy-Induced peripheral neurotoxicity in cancer survivors: predictors of Long-Term patient outcomes. J Natl Compr Canc Netw. 2021;19:821–8. - PubMed
-
- Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, et al. Prevention and management of Chemotherapy-Induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38:3325–48. - PubMed
-
- Hertz DL, Childs DS, Park SB, Faithfull S, Ke Y, Ali NT, McGlown SM, Chan A, Grech LB, Loprinzi CL, et al. Patient-centric decision framework for treatment alterations in patients with Chemotherapy-induced peripheral neuropathy (CIPN). Cancer Treat Rev. 2021;99:102241. - PubMed
-
- Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, Choi JH, Choi Y, Shim S, Lyu IS, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev. 2017;16:1160–73. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

