Lacc1-engineered extracellular vesicles reprogram mitochondrial metabolism to alleviate inflammation and cartilage degeneration in TMJ osteoarthritis
- PMID: 40186254
- PMCID: PMC11971819
- DOI: 10.1186/s12951-025-03355-5
Lacc1-engineered extracellular vesicles reprogram mitochondrial metabolism to alleviate inflammation and cartilage degeneration in TMJ osteoarthritis
Abstract
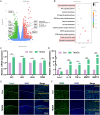
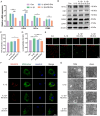
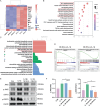
Temporomandibular joint osteoarthritis (TMJOA) is a multifaceted degenerative disease characterized by progressive cartilage degradation, chronic pain, and functional limitations of the TMJ, significantly affecting patients' quality of life. Although metabolic homeostasis in chondrocytes is crucial for cartilage health, the mechanisms underlying metabolic dysregulation in TMJOA remain poorly characterized. This study aimed to investigate the metabolic imbalance in TMJOA cartilage and explore novel therapeutic strategies targeting metabolic reprogramming. RNA sequencing revealed a significant imbalance between glycolysis and oxidative phosphorylation (OXPHOS) in TMJOA cartilage, with a marked shift toward glycolysis, which is associated with inflammation and cartilage degradation. To counteract this imbalance, Laccase domain-containing 1 (Lacc1), a metabolic regulator involved in both inflammation and metabolic homeostasis, was selected for investigation, as its role in chondrocytes had not been explored. We engineered macrophage-derived extracellular vesicles (EVs) to overexpress Lacc1 (OE-EVs), aiming to restore metabolic balance and modulate inflammation in chondrocytes. In vitro, OE-EVs significantly reduced IL-1β-induced inflammation, inhibited glycolysis by decreasing key glycolytic enzymes, improved mitochondrial function by decreasing mitochondrial superoxide levels, and the restoration of normal mitochondrial structure. In vivo, micro-computed tomography (Micro-CT) and histological analyses demonstrated that OE-EVs effectively alleviated inflammation and promoted cartilage repair, as indicated by a 1.55-fold increase in toluidine blue-stained cartilage area compared to the TMJOA group, reflecting improved cartilage matrix integrity and proteoglycan retention. These findings highlight the therapeutic potential of Lacc1-engineered EVs to target mitochondrial metabolism, reestablish metabolic homeostasis, and reduce inflammation in TMJOA, offering a novel and promising strategy for improving clinical outcomes in TMJOA patients.
Keywords: Chondrocytes; Engineered extracellular vesicles; Laccase domain-containing 1 (Lacc1); Mitochondrial metabolism; Temporomandibular joint osteoarthritis (TMJOA).
© 2025. The Author(s).
Conflict of interest statement
Author information. Ethics approval and consent to participate: All animal experimental protocols were approved by Animal Welfare Committee of Stomatological Hospital of Tongji University (Approved No. 2024-DW-19). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Wang XD, Zhang JN, Gan YH, Zhou YH. Current Understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94:666–73. - PubMed
-
- Delpachitra SN, Dimitroulis G. Osteoarthritis of the temporomandibular joint: a review of aetiology and pathogenesis. Br J Oral Maxillofac Surg. 2022;60:387–96. - PubMed
-
- Wang D, Qi Y, Wang Z, Guo A, Xu Y, Zhang Y. Recent advances in animal models, diagnosis, and treatment of temporomandibular joint osteoarthritis. Tissue Eng Part B. 2023;29:62–77. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

