Platycodon grandiflorum exosome-like nanoparticles: the material basis of fresh platycodon grandiflorum optimality and its mechanism in regulating acute lung injury
- PMID: 40186259
- PMCID: PMC11969861
- DOI: 10.1186/s12951-025-03331-z
Platycodon grandiflorum exosome-like nanoparticles: the material basis of fresh platycodon grandiflorum optimality and its mechanism in regulating acute lung injury
Abstract
Background: Acute lung injury (ALI) is a severe respiratory disease accompanied by diffuse inflammatory responses induced by various clinical causes. Many fresh medicinal plants have shown better efficacy than their dried forms in preventing and treating diseases like inflammation. As a classical Chinese herb, platycodon grandiflorum (PG) has been demonstrated effective in treating pneumonia, but most of previous studies focused on the efficacy of processed or dried PG formats, while the specific benefits of its fresh form are still underexplored. Exosome-like nanoparticles derived from medicinal plants are expected to point out an important direction for exploring the material basis and mechanism of this fresh herbal medicine.
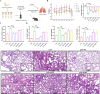
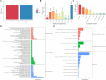
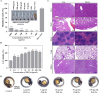
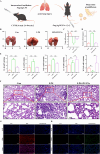
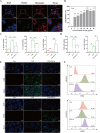
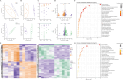
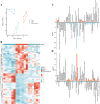
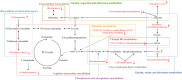
Results: The fresh form of PG could effectively improve ALI induced by lipopolysaccharide (LPS), relieve lung histopathological injury and weight loss, and reduce levels of inflammatory factors in mice, exhibiting better efficacy than dried PG in the treatment of ALI. Further extraction and purification of PG exosome-like nanoparticles (PGLNs) demonstrated that PGLNs had good biocompatibility, with characteristics consistent with general exosome-like nanoparticles. Besides, proteomic analysis indicated that PGLNs were rich in a variety of proteins. Animal experiments showed that PGLNs improved the pathological changes in LPS-induced lung tissues, inhibited the expression of inflammatory factors and promoted the expression of anti-inflammatory factors, and exerted a regulatory effect on the polarization of lung macrophages. Cell experiments further confirmed that PGLNs could be effectively taken up by RAW264.7 cells and repolarize M1 macrophages into M2 type, therefore reducing the secretion of harmful cytokines. Moreover, non-targeted metabolomics analysis reveals that PGLNs reduce inflammation and control macrophage polarization in a manner closely linked to pathways including glycolysis and lipid metabolism, highlighting a potential mechanism by which PGLNs protect the lungs from inflammatory damage like ALI.
Conclusion: Fresh PG has better anti-inflammatory and repair effects than its dried form. As one of the most effective active substances in fresh PG, PGLNs may regulate macrophage inflammation and polarization by regulating metabolic pathways including lipid metabolism and glycolysis, so as to reduce inflammation and repair lung injury.
Keywords: Acute lung injury; Fresh medicinal plant; Macrophage polarization; Non-targeted metabolomics; PGLNs; Platycodon grandiflorum.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Ethics Committee of the Center for Animal Experiments of Hunan University of Chinese Medicine (Approval number: SLBH-202307190001). Consent for publication: All authors have approved the manuscript and agree with the submission. Competing interests: The authors declare no competing interests.
Figures




References
-
- Meng J, Leung KS, Jiang Z, Dong X, Zhao Z. Establishment of GC-MS fingerprint of fresh houttuynia cordata. Chem Pharm Bull (Tokyo). 2005;53(11):1484–9. - PubMed
-
- Zheng Y, Lei L, Liang S, et al. Protective effect of fresh/dry dandelion extracts on APAP-Overdose-Induced acute liver injury. Chin J Integr Med. 2022;28(8):683–92. - PubMed
-
- Zhang L, Wang Y, Yang D, et al. Platycodon grandiflorus - an ethnopharmacological, phytochemical and Pharmacological review. J Ethnopharmacol. 2015;164:147–61. - PubMed
MeSH terms
Substances
Grants and funding
- S202410541014/National College Student Innovation and Entrepreneurship Training Program
- 2023JJ10031/Natural Science Foundation of Hunan Province
- kq2209019/Outstanding Innovative Youth Project of Changsha
- 82374266/National Natural Science Foundation of China
- 81973670/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

