Glucose absorption by isolated, vascularly perfused rat intestine: A significant paracellular contribution augmented by SGLT1 inhibition
- PMID: 40186371
- PMCID: PMC11971594
- DOI: 10.1111/apha.70033
Glucose absorption by isolated, vascularly perfused rat intestine: A significant paracellular contribution augmented by SGLT1 inhibition
Abstract
Aim: Intestinal glucose transport involves SGLT1 in the apical membrane of enterocytes and GLUT2 in the basolateral membrane. In vivo studies have shown that absorption rates appear to exceed the theoretical capacity of these transporters, suggesting that glucose transport may occur via additional pathways, which could include passive mechanisms. The aim of the study was to investigate glucose absorption in an in vitro model, which has proven useful for endocrine studies.
Methods: We studied both transcellular and paracellular glucose absorption in the isolated vascularly perfused rat small intestine. Glucose absorbed from the lumen was traced with 14C-d-glucose, allowing sensitive and accurate quantification. SGLT1 and GLUT2 activities were blocked with phlorizin and phloretin. 14C-d-mannitol was used as an indicator of paracellular absorption.
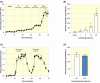
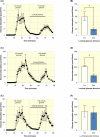
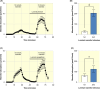
Results: Our results indicate that glucose absorption in this model involves two transport mechanisms: transport mediated by SGLT1/GLUT2 and a paracellular transport mechanism. Glucose absorption was reduced by 60% when SGLT1 transport was blocked and by 80% when GLUT2 was blocked. After combined luminal SGLT1 and GLUT2 blockade, ~30% of glucose absorption remained. d-mannitol absorption was greater in the proximal small intestine compared to the distal small intestine. Unexpectedly, mannitol absorption increased markedly when SGLT1 transport was blocked.
Conclusion: In this model, glucose absorption occurs via both active transcellular and passive paracellular transport, particularly in the proximal intestine, which is important for the understanding of, for example, hormone secretion related to glucose absorption. Interference with SGLT1 activity may lead to enhanced paracellular transport, pointing to a role in the regulation of the latter.
Keywords: glucose transporter 2; intestinal‐glucose‐absorption; intestine; paracellular transport; permeability; sodium glucose co‐transporter 1; transcellular transport.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

