Once-Weekly Insulin Versus Once-Daily Insulin for Type 1 Diabetes Treatment: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
- PMID: 40186384
- PMCID: PMC11971483
- DOI: 10.1002/edm2.70048
Once-Weekly Insulin Versus Once-Daily Insulin for Type 1 Diabetes Treatment: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
Background: Type 1 diabetes mellitus (T1DM) represents a considerable global health burden, affecting approximately 5%-10% of individuals with diabetes. Once-weekly basal insulin could substantially reduce the number of injections for T1DM patients from 365 daily to 52 weekly doses annually. Therefore, this meta-analysis compares the safety and efficacy of once-weekly insulin formulations.
Methods: The systematic review and meta-analysis included the relevant randomised controlled trials (RCTs) retrieved from PubMed, EMBASE, Web of Science, Cochrane, and SCOPUS databases until September 2024. The meta-analysis was performed using (RevMan 5.4.1). The study protocol was registered on PROSPERO (CRD42024603022).
Results: Three RCTs comprising 1724 participants were included. Once-daily insulin significantly decreased glycated haemoglobin (HbA1c) compared to once-weekly insulin (estimated treatment difference: 0.09%, 95% CI [0.07, 0.11], p < 0.00001). Fasting blood glucose levels were comparable between the once-weekly and once-daily insulin groups (estimated treatment difference: 0.44 mg/dL, 95% CI [-0.64, 1.52], p = 0.42). Once-weekly insulin was associated with a significant increase in the incidence of injection site reactions (RR: 3.48 with 95% CI [1.30, 9.31], p = 0.01), serious adverse events (RR: 1.55 with 95% CI [1.09, 2.19], p = 0.01), and treatment-emergent adverse events (RR: 1.12 with 95% CI [1.02, 1.23], p = 0.02), while no significant difference was observed in hypersensitivity reactions (RR: 1.04 with 95% CI [0.78, 1.38], p = 0.79).
Conclusion: Once-daily insulin has demonstrated slightly superior HbA1c reduction, while once-weekly insulin offers potential advantages in patient adherence. However, these benefits must be weighed against an increased risk of injection site reactions and nocturnal hypoglycemia. Although once-weekly insulin is more convenient, treatment decisions should consider individual patient factors such as hypoglycemia risk and tolerance to injection reactions.
Keywords: T1DM; diabetes mellitus; insulin; meta‐analysis.
© 2025 The Author(s). Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



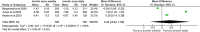



Similar articles
-
Efficacy and safety of once-weekly insulin versus once-daily insulin in patients with type 1 and type 2 diabetes mellitus: an updated meta-analysis of randomized controlled trials.Front Endocrinol (Lausanne). 2024 Nov 19;15:1459127. doi: 10.3389/fendo.2024.1459127. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39629047 Free PMC article.
-
Efficacy and safety of once-weekly insulin icodec in type 2 diabetes: A meta-analysis of ONWARDS phase 3 randomized controlled trials.Diabetes Obes Metab. 2024 Mar;26(3):1069-1081. doi: 10.1111/dom.15408. Epub 2024 Jan 8. Diabetes Obes Metab. 2024. PMID: 38192022
-
Comparative efficacy and safety of weekly tirzepatide versus weekly insulin in type 2 diabetes: A network meta-analysis of randomized clinical trials.Diabetes Obes Metab. 2024 Sep;26(9):3801-3809. doi: 10.1111/dom.15725. Epub 2024 Jun 25. Diabetes Obes Metab. 2024. PMID: 38923379
-
(Ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus.Cochrane Database Syst Rev. 2021 Mar 4;3(3):CD013498. doi: 10.1002/14651858.CD013498.pub2. Cochrane Database Syst Rev. 2021. PMID: 33662147 Free PMC article.
-
Clinical Outcomes With Once-Weekly Insulin Icodec Versus Once-Daily Insulin Glargine U100 in Insulin-Naïve and Previously Insulin-Treated Individuals With Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials.Endocrinol Diabetes Metab. 2024 May;7(3):e00480. doi: 10.1002/edm2.480. Endocrinol Diabetes Metab. 2024. PMID: 38659132 Free PMC article.
Cited by
-
Leonurine (SCM-198) exerts protective effects on pancreatic β-cells in type 1 diabetes by modulating the Bax/Bcl-2/Caspase-3 signaling pathway.BMC Complement Med Ther. 2025 Aug 14;25(1):306. doi: 10.1186/s12906-025-05051-1. BMC Complement Med Ther. 2025. PMID: 40813683 Free PMC article.
References
-
- CDC , “Diabetes Basics,” (2024), https://www.cdc.gov/diabetes/about/index.html.
-
- American Diabetes Association , “Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019,” Diabetes Care 42 (2019): S13–S28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

