Translating physiology of the arterial chemoreflex into novel therapeutic interventions targeting carotid bodies in cardiometabolic disorders
- PMID: 40186613
- PMCID: PMC12072261
- DOI: 10.1113/JP285081
Translating physiology of the arterial chemoreflex into novel therapeutic interventions targeting carotid bodies in cardiometabolic disorders
Abstract
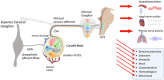
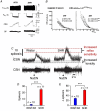
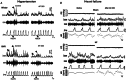
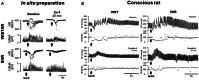
This review resulted from a conference on the pathological role of arterial chemoreflex and carotid bodies in cardiometabolic diseases held at the 27th Congress of the Polish Cardiac Society in September 2023 in Poznan, Poland. It reflects the contribution of Polish researchers and their international collaborations, which have been fundamental in the development of the field. Aberrant activity of the carotid bodies leads to both high tonicity and increased sensitivity of the arterial chemoreflex with resultant sympathoexcitation in chronic heart failure, resistant hypertension and obstructive sleep apnoea. This observation has led to several successful attempts of removing or denervating the carotid bodies as a therapeutic option in humans. Regrettably, such interventions are accompanied by serious respiratory and acid-base balance side-effects. Rather than a single stereotyped reaction, arterial chemoreflex comprises an integrative multi-system response to a variety of stimulants and its specific reflex components may be individually conveyed at varying intensities. Recent research has revealed that carotid bodies express diverse receptors, synthesize a cocktail of mediators, and respond to a plethora of metabolic, hormonal and autonomic nervous stimuli. This state-of-the-art summary discusses exciting new discoveries regarding GLP-1 receptors, purinergic receptors, the glutamate-GABA system, efferent innervation and regulation of blood flow in the carotid body and how they open new avenues for novel pharmacological treatments selectively targeting specific receptors, mediators and neural pathways to correct distinct responses of the carotid body-evoked arterial chemoreflex in cardiometabolic diseases. The carotid body offers novel and advantageous therapeutic opportunities for future consideration by trialists.
Keywords: carotid body; diabetes mellitus; heart failure; hyperglycaemia; hypertension; hypoxia; sleep apnoea; sympathetic nervous system.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
Authors declare no conflict of interest to this report.
Figures



References
-
- Abdala, A. P. , McBryde, F. D. , Marina, N. , Hendy, E. B. , Engelman, Z. J. , Fudim, M. , Sobotka, P. A. , Gourine, A. V. , & Paton, J. F. R. (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. The Journal of Physiology, 590(17), 4269–4277. - PMC - PubMed
-
- Accorsi‐Mendonca, D. , Castania, J. A. , Bonagamba, L. G. H. , Machado, B. H. , & Leao, R. M. (2011). Synaptic profile of nucleus tractus solitarius neurons involved with the peripheral chemoreflex pathways. Neuroscience, 197, 107–120. - PubMed
-
- Alvarez‐Buylla, R. , & de Alvarez‐Buylla, E. R. (1988). Carotid sinus receptors participate in glucose homeostasis. Respiration Physiology, 72(3), 347–359. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
