Lamin A/C regulates cerebellar granule cell maturation
- PMID: 40186700
- PMCID: PMC11972193
- DOI: 10.1007/s10565-025-10011-z
Lamin A/C regulates cerebellar granule cell maturation
Abstract
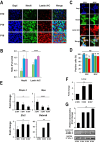
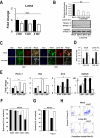
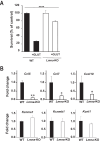
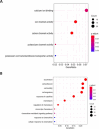
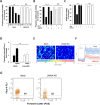
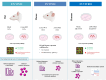
Lamin A/C is a nuclear type V intermediate filament protein part of the meshwork structure underlying the inner nuclear membrane (nuclear lamina), which plays numerous roles, including maintenance of nuclear shape, heterochromatin organization, and transcriptional regulation. Our group has demonstrated the role of Lamin A/C in different pathophysiological conditions. Here, we investigated for the first time how Lamin A/C affects neuronal maturation in rat cerebellar granule cells (GCs). Primary rat cerebellar GCs where we silenced the Lmna gene constituted our key model; this provided a rather homogeneous cellular system showing a neuronal population in vitro. We then validated our findings in another in vivo murine model with knock-out of the Lmna gene and in an in vitro human neuronal model with silencing of the LMNA gene. We observed across three different models that Lamin A/C down-regulation affects neurons maturation by protecting the cells from glutamate-evoked excitotoxicity and correlates with an inhibition of calcium influxes and a down-regulation of pro-inflammatory cytokine pathways. Consistent with previous findings from our group, this study corroborates that Lamin A/C plays a key role in neural development and opens new significant implications for a better comprehension of the mechanisms involved in neurodegenerative diseases, where changes in the nuclear envelope are linked to neuroinflammatory processes and damage.
Keywords: Lamin A/C; Neuronal development; Cerebellar granule cells; Glutamate; Neurotoxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Alieh LHA and Herrera A. Heterogeneity and developmental dynamics of mammalian neocortical progenitors. Current Opinion Syst Biol. 2023;32–33:100444. 10.1016/j.coisb.2023.100444.
-
- Amadoro G, Serafino AL, Barbato C, Ciotti MT, Sacco A, Calissano P, Canu N. Role of N-terminal tau domain integrity on the survival of cerebellar granule neurons. Cell Death Differ. 2004;11:217–30. 10.1038/sj.cdd.4401314. - PubMed
-
- Amadoro G, Pieri M, Ciotti MT, Carunchio I, Canu N, Calissano P, Zona C, Severini C. Substance P provides neuroprotection in cerebellar granule cells through Akt and MAPK/Erk activation: evidence for the involvement of the delayed rectifier potassium current. Neuropharmacology. 2007;52(6):1366–77. 10.1016/j.neuropharm.2007.01.020. - PubMed
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–73. 10.1016/0896-6273(95)90186-8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

