Health-Related Quality of Life in Chronic Hand Eczema in a Phase 2b Trial of Delgocitinib Cream
- PMID: 40186746
- PMCID: PMC12033156
- DOI: 10.1007/s13555-025-01384-4
Health-Related Quality of Life in Chronic Hand Eczema in a Phase 2b Trial of Delgocitinib Cream
Abstract
Introduction: Chronic Hand Eczema (CHE) is a multifactorial, burdensome, inflammatory skin disease, with limited treatment options. In a double-blind dose-ranging phase 2b clinical trial, participants with CHE received delgocitinib cream, a topical pan-Janus kinase (JAK) inhibitor, or cream vehicle (clinical results published elsewhere). The objectives were to analyse patient-reported outcomes (PROs) in participants with mild and moderate to severe CHE at screening, and to investigate the impact on PROs during treatment in participants with moderate to severe CHE.
Methods: Firstly, Dermatology Life Quality Index (DLQI), EQ-5D-5L, and Hand Eczema Impact Scale (HEIS) per severity were analysed at screening. Secondly, PROs were analysed in the subset of participants with moderate to severe CHE; participants receiving delgocitinib cream 20 mg/g were compared with participants receiving cream vehicle for 16 weeks.
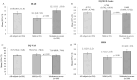
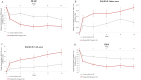
Results: At screening, mean (SD) DLQI, EQ-5D-5L, and HEIS were 8.1 (5.8), 0.788 (0.175), and 1.7 (0.8), respectively for mild CHE (n = 93), and 12.1 (6.9), 0.689 (0.236), and 2.3 (0.9) for participants with moderate to severe CHE (n = 202), respectively. Among the participants with moderate to severe CHE who received delgocitinib (n = 41), the least squares mean [SE] change from baseline to week 16 improved compared to cream vehicle (n = 38) in DLQI (- 7.1 [0.9] vs. - 4.6 [0.9]), EQ-5D-5L (0.228 [0.032] vs. 0.096 [0.034]), and HEIS (- 1.5 [0.2] vs. - 0.8 [0.2]) (P < 0.05).
Conclusions: Mild CHE had a moderate effect, whereas moderate to severe CHE had a very large effect on patients' Health-Related Quality of Life at screening. Treatment with delgocitinib cream was associated with considerable improvement in PROs and represents a potentially valuable treatment option.
Trial registration: ClinicalTrials. gov identifier NCT03683719.
Keywords: Burden of disease; Chronic Hand Eczema; Contact dermatitis; Delgocitinib cream; Health-related quality of life; Patient-reported outcomes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Timo Buhl has been a speaker/investigator/advisor for AbbVie, ALK, Almirall, AstraZeneca, Bencard, Eli Lilly, Incyte, Galderma, Janssen, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, R-Biopharm, Sanofi and ThermoFisher. Andrea Bauer has been a speaker/advisor/investigator and/or received research funding from AbbVie, Almirall, Amgen, AstraZeneca, Biofrontera, Bristol-Myers Squibb, Celldex, Eli Lilly, Escient, Galderma, Genentech, Gilead, Incyte, Janssen, Jasper, LEO Pharma, L’Oréal, Novartis, Pfizer, Pharvaris, Sanofi/Regeneron and Shire/Takeda. Benjamin D. Ehst has been a speaker/advisor/investigator and/or received research funding from AbbVie, Acelyrin, Aclaris, Allakos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert Pharma, Dermavant Sciences, Eli Lilly, Evelo Biosciences, Evommune, Incyte, Janssen, Kymab, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, Takeda, UCB Pharma and Ventyx. Julie Hahn-Pedersen was an employee of LEO Pharma A/S, Ballerup, Denmark at the time of the study and is currently employed by Novo Nordisk A/S. Berith Fredsted Hagen was an employee of LEO Pharma A/S, Ballerup, Denmark at the time of the study and is currently employed by Zealand Pharma. Jacob P. Thyssen was an employee at the Department of Dermatology and Venereology, Bispebjerg Hospital, University of Copenhagen, Denmark at the time of the study and is currently employed at LEO Pharma A/S, Ballerup, Denmark. Eydna D. Apol is an employee at LEO Pharma A/S, Ballerup, Denmark. Tove Agner has been a speaker/investigator/advisor for AbbVie, Almirall, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme. Ethical Approval: The trial was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments, Good Clinical Practice guidelines, and applicable regulatory requirements, and was approved by the Regional Committees on Health Research Ethics for Capital Region of Denmark (reference number 18047858), and independent ethics committees or institutional review boards for each study site (supplementary material). All patients provided written informed consent.
Figures
References
-
- Buhl T, Bauer A, Thyssen JP, Hahn-Pedersen J, Hagen BF, Agner T. Treatment with delgocitinib cream improves health-related quality of life in patients with chronic hand eczema. In: 15th congress of the European Society of Contact Dermatitis (ESCD), 8–10 June 2022. Amsterdam, the Netherlands: Contact Dermatitis; 2022.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

