Overall survival of palbociclib plus endocrine therapy in Japanese patients with HR+/HER2- advanced breast cancer in the first-or second-line setting: a multicenter observational study (P-BRIDGE study)
- PMID: 40186791
- PMCID: PMC12174214
- DOI: 10.1007/s12282-025-01689-4
Overall survival of palbociclib plus endocrine therapy in Japanese patients with HR+/HER2- advanced breast cancer in the first-or second-line setting: a multicenter observational study (P-BRIDGE study)
Erratum in
-
Correction: Overall survival of palbociclib plus endocrine therapy in Japanese patients with HR+/HER2- advanced breast cancer in the first-or second-line setting: a multicenter observational study (P-BRIDGE study).Breast Cancer. 2026 Jan 27. doi: 10.1007/s12282-025-01787-3. Online ahead of print. Breast Cancer. 2026. PMID: 41591707 No abstract available.
Abstract
Background: Recently, we reported the real-world effectiveness of palbociclib plus endocrine therapy (ET) in HR+/HER2- advanced breast cancer (ABC) in Japan (NCT05399329). However, median overall survival (OS) was not reached because of limited follow-up (36 months). Here, we present follow-up data from this study, including real-world clinical outcomes and treatment patterns.
Methods: The P-BRIDGE study was a multi-center, observational study evaluating the real-world effectiveness and treatment patterns of patients diagnosed with HR+/HER2- ABC who received palbociclib plus ET in first (1L) or second line (2L) in Japan. The primary endpoint was real-world progression-free survival (rwPFS); secondary endpoints included OS and chemotherapy-free survival (CFS).
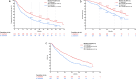
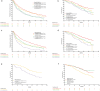
Results: Of the 693 eligible patients, 426 and 267 patients received palbociclib with ET as 1L and 2L treatment, respectively. After a median follow-up of 48.1 months, the median rwPFS (95% CI) was 26.2 months (21.4-30.4) for 1L and 14.9 months (11.7-18.3) for 2L, respectively. Median OS (95% CI) was 68.2 months (60.8-NE) for 1L and 50.7 months (42.2-57.2) for 2L, respectively. OS analysis was also performed in the following subgroups: TFI < 12 months/TFI ≥ 12months/de novo metastatic median OS was 56.3 months (43.9-68.2), NR (NE-NE), NR (56.3-NE), visceral metastasis was 65.0 months (56.3-NE), liver metastasis was 46.4 months (37.2-NE), and bone only metastasis was NR (57.8-NE) in 1L, respectively.
Conclusions: The updated results from this study further confirm the real-world effectiveness of palbociclib plus ET in routine clinical practice in Japan. More than 5 years of median OS in 1L was observed, supporting the use of palbociclib plus ET as 1L standard of care for HR+/HER2- ABC.
Keywords: Advanced breast cancer; CDK4/6 inhibitors; Japanese patients; Overall survival; Palbociclib; Real-world evidence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: SN reports honoraria from AstraZeneca, Daiichi-Sankyo, Eisai, Eli Lilly, MSD, Taiho, Chugai, and Pfizer. MaHa reports honoraria from Eli Lilly and Pfizer.TY reports honoraria from AstraZeneca, Chugai, Eisai, Eli Lilly, MSD, and Pfizer. HiMa reports honoraria from Eli Lilly, Chugai, and Pfizer. KW reports honoraria from AstraZeneca, Daiichi-Sankyo, Eisai, Shionogi, Kyowa-Kirin, Nippon-Kayaku, Novartis, Eli Lilly, Taiho, Chugai, and Pfizer. TN reports honoraria from AstraZeneca, Daiichi-Sankyo, Eisai, Novartis, Eli Lilly, Taiho, Chugai, and Pfizer. TS reports honoraria from Chugai, Novartis, MSD, Taiho, Eisai, Maruho, PDRadiopahrma, Nippon-Kayaku, Kyowa-Kirin, AstraZeneca, Eli Lilly, Daiichi -Sankyo and Pfizer; and institutional support from Taiho. MY reports honoraria from AstraZeneca, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Nippon-Kayaku, Eli Lilly, Taiho, Chugai, and Pfizer. TI reports honoraria from Chugai, and Pfizer. MF reports honoraria from Chugai, Taiho, Eisai, Nippon-Kayaku, Eli Lilly, and Daiichi -Sankyo. NK is employees of Pfizer Inc. is stockholders in Pfizer Inc. YM is employees of Pfizer Inc. is stockholders in Pfizer Inc. NM reports honoraria from Chugai, Pfizer, AstraZeneca, Eli Lilly, and Daiichi -Sankyo; and institutional support from Chugai, Eli Lilly, AstraZeneca, Pfizer, Daiichi-Sankyo, MSD, Eisai, Novartis, Sanofi, KyowaKirin, and Nippon Kayaku. TO, MT, DT, MiHa, HY, NaMo, HY, CO, MI, and SS have no disclosures.
Figures
References
-
- Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29. - DOI - PMC - PubMed
-
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

