Minimally invasive, wide-field two-photon imaging of the brainstem at cellular resolution
- PMID: 40187350
- PMCID: PMC12256953
- DOI: 10.1016/j.crmeth.2025.101010
Minimally invasive, wide-field two-photon imaging of the brainstem at cellular resolution
Abstract
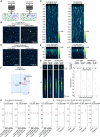
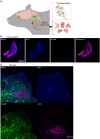
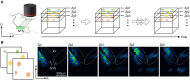
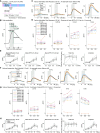
Brain-viscera communication is crucial for regulating mental health, with the vagus nerve being a key structure mediating this interaction. Clinically, artificial vagus nerve stimulation (VNS) is used to treat various neuropsychiatric disorders, highlighting the importance of vagal afferent fibers in emotion regulation. The nucleus tractus solitarii (NTS) is a brainstem structure proposed to receive signals from vagal afferents and relay them to brain networks for emotion regulation. However, due to the anatomical complexity and difficulty in accessing the deep-brain NTS region in vivo, its underlying mechanisms remain unclear. Here, we developed a wide-field and deep-brain two-photon imaging method using a double-prism optical interface. This approach enables cellular-resolution imaging to specifically detect NTS neural activity while largely preserving the overlying cerebellum, a region also implicated in emotion regulation. We evaluated NTS neuronal responses to VNS and a gastrointestinal hormone, demonstrating the method's utility for investigating the vagus-NTS pathway in vivo.
Keywords: CP: Imaging; CP: Neuroscience; brain-viscera communication; brainstem; emotion; in vivo two-photon calcium imaging; nucleus tractus solitarii; vagus nerve stimulation.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Khalsa S.S., Adolphs R., Cameron O.G., Critchley H.D., Davenport P.W., Feinstein J.S., Feusner J.D., Garfinkel S.N., Lane R.D., Mehling W.E., et al. Interoception and Mental Health: A Roadmap. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2018;3:501–513. doi: 10.1016/j.bpsc.2017.12.004. - DOI - PMC - PubMed
-
- Chen W.G., Schloesser D., Arensdorf A.M., Simmons J.M., Cui C., Valentino R., Gnadt J.W., Nielsen L., Hillaire-Clarke C.S., Spruance V., et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021;44:3–16. doi: 10.1016/j.tins.2020.10.007. - DOI - PMC - PubMed
-
- Yamada T., Katagiri H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr. J. 2007;54:497–505. doi: 10.1507/endocrj.kr-106. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

