Twice against thrice-weekly hemodialysis (TATH): a multicenter nonrandomized trial
- PMID: 40188011
- PMCID: PMC11972488
- DOI: 10.1186/s12882-025-04105-3
Twice against thrice-weekly hemodialysis (TATH): a multicenter nonrandomized trial
Abstract
Background: The optimal frequency of maintenance hemodialysis remains a subject of debate. In many countries, twice-weekly hemodialysis is still commonly practiced. This trial aimed to compare the outcomes of patients undergoing twice-weekly versus thrice-weekly hemodialysis.
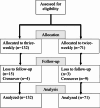
Methods: This prospective, multicenter, nonrandomized trial included incident adult patients, with chronic kidney disease stage 5, initiating hemodialysis between January 2018 and August 2021. Patients were allocated to either a twice-weekly or thrice-weekly regimen, and monitored at 1, 3, 6, 12 and 24 months. This trial was terminated before reaching the required sample size due to the COVID-19 pandemic and economic factors. Recruitment achieved 25% of the projected number. Missing baseline factors were imputed using multiple imputation algorithms, then entered in a logistic regression model to estimate propensity scores. The primary outcome was two-year survival analyzed using a Cox regression survival model adjusted for propensity scores and baseline residual urine output. Secondary outcomes included hospitalization rates, uncontrolled hypertension and cumulative erythropoietin dose at two years, analyzed using regression models adjusted for propensity scores and baseline residual urine output. All analyses were conducted on an intention-to-treat basis.
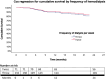
Results: A total of 132 patients on thrice-weekly hemodialysis and 71 on twice-weekly hemodialysis were included. The mean age was 67 ± 15 years and the median eGFR at dialysis initiation was 6 (4,8) mL/min/1.73 m2. At one year, patients in the twice-weekly group had greater residual urine output. At two years, there was no significant difference in survival (HR = 0.84; 95% CI: 0.37, 1.90), hospitalization rates (P = 0.515) or uncontrolled hypertension (P = 0.442). The twice-weekly group showed a trend toward higher erythropoietin requirements (P = 0.08). Serum potassium levels and the number of antihypertensive medications were greater in the twice-weekly group.
Conclusions: Patients on twice-weekly hemodialysis showed comparable overall survival at two years to those on thrice-weekly hemodialysis. While a twice-weekly regimen may be a viable option during the first year of dialysis, especially in low-resource settings, it carries potential risks that necessitate careful monitoring after the first year.
Trial registration: The trial was registered on ClinicalTrials.gov on January 16, 2018 (Identifier NCT03415776).
Keywords: Antihypertensive drugs; Erythropoietin; Frequency; Hemodialysis; Mortality; Residual diuresis; Residual urine output; Serum potassium; Thrice-weekly; Twice-weekly.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the ethics committee of Hotel Dieu de France/Saint-Joseph University (CEHDF 1114) and was conducted in agreement with the Helsinki Declaration of 1975. All included patients gave their written informed consent. Consent for publication: All patients gave their consent for results’ dissemination. Study registration: The trial was registered on ClinicalTrials.gov (Identifier NCT03415776) on January 16, 2018. Competing interests: The authors declare no competing interests.
Figures
References
-
- Lameire N, Van Vanholder BW. Did 20 years of technological innovations in Hemodialysis contribute to better patient outcomes?? Clin J Am Soc Nephrol. 2009;4:S30–40. - PubMed
-
- Hanson JA, Hulbert-Shearon TE, Ojo AO, Port FK, Wolfe RA, Agodoa LY, Daugirdas JT. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625– 33. 10.1159/000013533. PMID: 10592355. - PubMed
-
- Tattersall J, Martin-Malo A, Pedrini L, et al. EBPG guideline on Dialysis strategies. Nephrol Dial Transpl. 2007;22(Suppl 2):ii5–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous