Discontinuation vs. continuation of concomitant methotrexate in patients with rheumatoid arthritis on certolizumab pegol: results from a randomised, controlled trial
- PMID: 40188084
- PMCID: PMC11972473
- DOI: 10.1186/s13075-025-03548-1
Discontinuation vs. continuation of concomitant methotrexate in patients with rheumatoid arthritis on certolizumab pegol: results from a randomised, controlled trial
Abstract
Objective: The present non-inferiority study was designed to compare the effect of discontinuing versus continuing methotrexate (MTX) alongside certolizumab pegol (CZP) on maintaining low disease activity (LDA) in rheumatoid arthritis (RA) patients already stable on combination therapy.
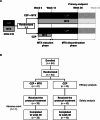
Methods: This multicentre, open-label, randomised, controlled trial included RA patients with sustained LDA (Clinical Disease Activity Index [CDAI] ≤ 10) for ≥ 12 weeks with CZP + MTX. Patients were randomised 1:1 by computer to either continue MTX (CZP + MTX group) or discontinue MTX after a 12-week reduction period (CZP group) using a dynamic allocation strategy with the minimisation method. The primary endpoint was the proportion of patients maintaining LDA without a flare (i.e., a CDAI score > 10 or intervention with rescue treatments for any reason) at week 36 (24 weeks after MTX discontinuation). Non-inferiority is verified if the lower limit of the 90% confidence interval (CI) using normal approximation for the difference in the proportion of cases that maintained LDA at week 36 between the intervention group and control group exceeds the non-inferiority margin.
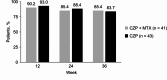
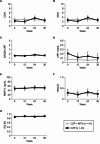
Results: All 84 screened patients were randomised to the CZP + MTX group (n = 41) and CZP group (n = 43), and were included in the efficacy analysis. Proportions (90% CI) of patients who maintained LDA at week 36 were 85.4% (76.3 to 94.4%) in the CZP + MTX group and 83.7% (74.5 to 93.0%) in the CZP group. The difference (90% CI) between the two groups was - 1.6% (-14.6 to 11.3%), with the lower limit of the 90% CI exceeding the non-inferiority margin of -18%. Reported adverse events were broadly similar between the two groups. The proportion of patients with gastrointestinal symptoms, as assessed by a self-administered questionnaire, was significantly lower in the CZP group than in the CZP + MTX group at week 36 (2.4% vs. 15.8%, P = 0.034).
Conclusion: Discontinuing concomitant MTX in RA patients on CZP is clinically feasible for maintaining LDA.
Trial registration: Japan Registry of Clinical Trials (jRCTs041200048).
Keywords: Certolizumab pegol; Drug tapering; Methotrexate; Randomised controlled trial; Rheumatoid arthritis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol was centrally reviewed and approved by the Certified Review Board of the Nagoya University Graduate School of Medicine (2020 − 0303), and was registered with the Japan Registry of Clinical Trials (jRCTs041200048). The present study was conducted in accordance with the Clinical Trials Act, and complied with the Declaration of Helsinki. Written informed consent was obtained from all patients. Consent for publication: Not applicable. Competing interests: SA has received grant/research support, consulting fees, and/or speakers’ fees from AbbVie, Asahi Kasei, Astellas, Ayumi, Chugai, Eisai, Eli Lilly, Taisho, and UCB Japan. TK has received grant/research support and/or speakers’ fees from AbbVie, Astellas, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, and Pfizer. HH has received grant/research support and/or speakers’ fees from Japan Research Foundation Clinical Pharmacology, AbbVie, Asahi Kasei, Astellas, AstraZeneca, Bristol-Myers Squibb, Eisai, GlaxoSmithKline, Nihon Pharmaceutical, Ono, and Taisho. YK has received speakers’ fees from Asahi Kasei, Astellas, Eisai, and Eli Lilly. YH has received speakers’ fees from Astellas and UCB Japan. TH has received speakers’ fees from AbbVie, Asahi Kasei, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Janssen, and Pfizer. HY has received grant/research support and/or speakers’ fees from AbbVie, Asahi Kasei, Astellas, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Kissei, Janssen, Nippon Shinyaku, Mitsubishi Tanabe, Novartis, Pfizer, Sanofi, Taisho, Takeda, Teijin, and Viatris. YK has received speakers’ fees from Astellas. KM has received speakers’ fees from Mitsubishi Tanabe and Pfizer, and is affiliated with an endowed department sponsored by Ayumi, Mitsubishi Tanabe, and UCB Japan. KM has received speakers’ fees from AbbVie, Asahi Kasei, Astellas, Boehringer Ingelheim, Chugai, Eisai, Eli Lilly, Gilead, Mitsubishi Tanabe, Mochida, Novartis, Ono, Pfizer, Taisho, Takeda, UCB Japan, and Viatris. MS has received speakers’ fees from AbbVie, Asahi Kasei, Astellas, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Mitsubishi Tanabe, Novartis, Pfizer, Sanofi, Taisho, Takeda, and UCB Japan. KT has received grant/research support and/or speakers’ fees from AbbVie, Asahi Kasei, Astellas, Chugai, Eisai, Gilead, Japan Rheumatism Foundation, Mitsubishi Tanabe, Novo Nordisk, Pfizer, Sanofi, and UCB Japan. All other authors declare no conflicts of interest.
Figures

References
-
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–81. - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. - PubMed
-
- Kaneko Y, Atsumi T, Tanaka Y, Inoo M, Kobayashi-Haraoka H, Amano K, et al. Comparison of adding Tocilizumab to methotrexate with switching to Tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis. 2016;75(11):1917–23. - PMC - PubMed
-
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Results of a two-year followup study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis Rheum. 2008;58(4):953–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

