Efficacy of gemcitabine plus nab-paclitaxel in second-line treatment of metastatic pancreatic cancer
- PMID: 40188172
- PMCID: PMC11972389
- DOI: 10.1038/s41598-025-96157-6
Efficacy of gemcitabine plus nab-paclitaxel in second-line treatment of metastatic pancreatic cancer
Abstract
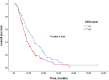
Despite numerous studies on second-line therapies in metastatic pancreatic cancer, there is no randomized study evaluating the efficacy of gemcitabine plus nab-paclitaxel as a second-line treatment. This study aims to examine the efficacy of gemcitabine plus nab-paclitaxel in second-line therapy. In this retrospective study, a total of 218 patients from 23 centers were included. The primary endpoint was progression-free survival (PFS), secondary endpoints included overall survival (OS), treatment efficacy based on ECOG performance status (PS), and tumor marker (CEA, CA 19 - 9) levels. In the second-line treatment with gemcitabine plus nab-paclitaxel, the median PFS was 5.1 months (95% CI, 5.6 to 7.1), and the median OS was 8.6 months (95% CI, 7.3 to 10.0). Median PFS was 6.6 months in patients with normal CEA levels compared to 4.4 months in patients with high CEA levels (P = 0.01). Median PFS was 6 months in patients with ECOG PS 0-1 compared to 3.8 months in patients with PS 2 (P < 0.01). This study demonstrates the contribution of gemcitabine plus nab-paclitaxel in both OS and PFS in second-line treatment of metastatic pancreatic cancer. It was found to be a good option especially for young patients with good ECOG PS.
Keywords: Gemcitabine plus Nab-Paclitaxel; Metastatic pancreatic cancer; Overall survival; Progression-free survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and informed consent: The study protocol was approved by The Van Regional Training and Research Hospital (IRB No. 2023 / 19/ 04 -13.09.2023). Due to the retrospective nature of the study, the need for informed consent was waived with the decision of the Ethics Committee of Van Regional Training and Research Hospital. The study was performed in accordance with the ethical guidelines of the World Medical Association’s Declaration of Helsinki.
Figures





References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin.74, 229–263 (2024). - PubMed
-
- Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med.364, 1817–1825 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

