Tobacco smoke exposure is a driver of altered oxidative stress response and immunity in head and neck cancer
- PMID: 40188338
- PMCID: PMC11971752
- DOI: 10.1186/s12967-025-06258-z
Tobacco smoke exposure is a driver of altered oxidative stress response and immunity in head and neck cancer
Abstract
Background: Exposomes are critical drivers of carcinogenesis. However, how they modulate tumor behavior remains unclear. Extensive clinical data show cigarette smoke to be a key exposome that promotes aggressive tumors, higher rates of metastasis, reduced response to chemoradiotherapy, and suppressed anti-tumor immunity. We sought to determine whether smoke itself can modulate aggressive tumor behavior in head and neck squamous cell carcinoma (HNSCC) through reprogramming of the cellular reductive state.
Methods: Using established human and murine HNSCC cell lines and syngeneic mouse models, we utilized conventional western blotting, steady state and flux metabolomics, RNA sequencing, quantitative proteomics and flow cytometry to analyze the impact of smoke exposure on HNSCC tumor biology and anti-tumor immunity.
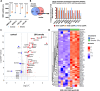
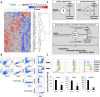
Results: Cigarette smoke persistently activated Nrf2 target genes essential for maintenance of the cellular reductive state and survival under conditions of increased oxidative stress in HNSCC regardless of human papillomavirus (HPV) association. In contrast to e-cigarette vapor, conventional cigarette smoke mobilizes cellular metabolism toward oxidative stress adaptation, resulting in development of cross-resistance to cisplatin. In parallel, smoke exposure modulates expression of PDL1 and the secretory phenotype of HNSCC cells resulting in an altered tumor immune microenvironment (TIME) in syngeneic mouse models and downregulated expression of antigen presentation and costimulatory genes in myeloid cells.
Conclusion: The cigarette smoke exposome is a potent activator of the Nrf2 pathway and appears to be the primary trigger for a tripartite phenotype of aggressive HNSCC consisting of: (1) reduced chemotherapy sensitivity, (2) enhanced metastatic potential and (3) suppressed anti-tumor immunity.
Keywords: Glutathione; Keap1; Nrf2; Oxidative stress; PDL1; Tobacco.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal work performed in the current manuscript was in accordance with the rules of the Baylor College of Medicine Institutional Animal Care and Use Committee. Consent for publication: Not applicable. Competing interests: The authors report no conflicts of interest relevant to the work summarized in the current manuscript. V.C.S. is a consultant and equity holder in Femtovox Inc. and a consultant for PDS Biotechnology. S.Y.L. is a medical affairs consultant for Cardinal Health. W.K.D. holds an ownership stake in and is a paid advisor of the Diakonos Oncology Corporation. W.K.D also disclosed his role as a paid advisor of APAC Biotech, Pvt, Ltd from 2015 to 2020. All other authors declare no conflicts of interest. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Figures






Update of
-
Tobacco smoke exposure is a driver of altered oxidative stress response and immunity in head and neck cancer.bioRxiv [Preprint]. 2024 Oct 21:2024.10.17.618907. doi: 10.1101/2024.10.17.618907. bioRxiv. 2024. Update in: J Transl Med. 2025 Apr 05;23(1):403. doi: 10.1186/s12967-025-06258-z. PMID: 39484602 Free PMC article. Updated. Preprint.
References
-
- Fakhry C, Zhang Q, Gillison ML, Nguyen-Tan PF, Rosenthal DI, Weber RS, Lambert L, Trotti AM 3rd, Barrett WL, Thorstad WL, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer. 2019;125:2027–38. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

