Immunological consequences of CAR T-cell therapy: an analysis of infectious complications and immune reconstitution
- PMID: 40188456
- PMCID: PMC12242403
- DOI: 10.1182/bloodadvances.2024015346
Immunological consequences of CAR T-cell therapy: an analysis of infectious complications and immune reconstitution
Abstract
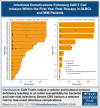
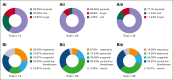
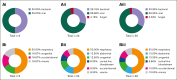
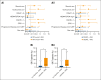
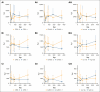
Chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy in treating relapsed and refractory (R/R) B-cell neoplasms, such as diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM). Despite its success, the long-term effects and sequelae of CAR T cells on the immune system remain underexplored. This study presents a 1-year follow-up analysis of 52 patients (42 with R/R DLBCL and 10 with R/R MM) treated with anti-CD19- and B-cell maturation antigen-targeted CAR T cells, focusing on immune reconstitution and infectious complications. Our findings reveal that CAR T-cell therapy leads to profound depletion of B and T cells. CD4+ T cells and CD19+ B cells exhibited impaired regeneration after treatment. Infections were more frequent during the first 30 days. In the short-term follow-up, density of infections within 100 days at risk was 1.8 in patients with DLBCL and 4.6 in patients with MM, with bacterial infections predominating in this early period after CAR T-cell infusion. In addition, we observed a shift to viral infections in the long-term follow-up, alongside with a decline in infection density to 0.1 in patients with DLBCL and 0.4 infections per 100 days at risk in patients with MM, respectively. Severe cytokine release syndrome was associated with a higher risk of late-onset infections. These findings highlight the importance of close monitoring and prophylactic measures in patients undergoing CAR T-cell therapy to reduce infection risks and enhance immune recovery.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: W.B. reports consulting for Janssen. A.R. and B.B. report receiving honoraria from Janssen. The remaining authors declare no competing financial interests.
Figures

References
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. - PubMed
-
- Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-cell therapy in autoimmune disease — a case series with follow-up. N Engl J Med. 2024;390(8):687–700. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

