Decoding ischemic stroke: Perspectives on the endoplasmic reticulum, mitochondria, and their crosstalk
- PMID: 40188640
- PMCID: PMC12001122
- DOI: 10.1016/j.redox.2025.103622
Decoding ischemic stroke: Perspectives on the endoplasmic reticulum, mitochondria, and their crosstalk
Abstract
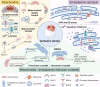
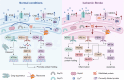
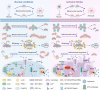
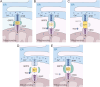
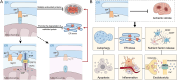
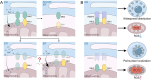
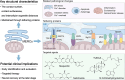
Stroke is known for its high disability and mortality rates. Ischemic stroke (IS), the most prevalent form, imposes a considerable burden on affected individuals. Nevertheless, existing treatment modalities are hindered by limitations, including narrow therapeutic windows, substantial adverse effects, and suboptimal neurological recovery. Clarifying the pathological mechanism of IS is a prerequisite for developing new therapeutic strategies. In this context, the functional disruption of mitochondria, the endoplasmic reticulum (ER), and the crosstalk mechanisms between them have garnered increasing attention for their contributory roles in the progression of IS. Therefore, this review provides a comprehensive summary of the current pathomechanisms associated with the involvement of the ER and mitochondria in IS, emphasising Ca2+ destabilization homeostasis, ER stress, oxidative stress, disordered mitochondrial quality control, and mitochondrial transfer. Additionally, this article highlights the functional interaction between the ER and mitochondria, as well as the mitochondrial-ER contacts (MERCs) that structurally connect mitochondria and the ER, aiming to provide ideas and references for the research and treatment of IS.
Keywords: Endoplasmic reticulum; Ischemic stroke; Mitochondria; Mitochondria and endoplasmic reticulum contacts; Oxidative stress.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest.
Figures


References
-
- Röther J., Ford G.A., Thijs V.N.S. Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities. Cerebrovasc. Dis. 2013;35(4):313–319. - PubMed
-
- Maiorov S.A., Kairat B.K., Berezhnov A.V., Zinchenko V.P., Gaidin S.G., Kosenkov A.M. Peculiarities of ion homeostasis in neurons containing calcium-permeable AMPA receptors. Arch. Biochem. Biophys. 2024;754 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

