Wolfram syndrome 2 gene (CISD2) deficiency disrupts Ca2+-mediated insulin secretion in β-cells
- PMID: 40189101
- PMCID: PMC12020879
- DOI: 10.1016/j.molmet.2025.102140
Wolfram syndrome 2 gene (CISD2) deficiency disrupts Ca2+-mediated insulin secretion in β-cells
Abstract
Objective: Diabetes, characterized by childhood-onset, autoantibody-negativity and insulin-deficiency, is a major manifestation of Wolfram syndrome 2 (WFS2), which is caused by recessive mutations of CISD2. Nevertheless, the mechanism underlying β-cell dysfunction in WFS2 remains elusive. Here we delineate the essential role of CISD2 in β-cells.
Methods: We use β-cell specific Cisd2 knockout (Cisd2KO) mice, a CRISPR-mediated Cisd2KO MIN6 β-cell line and transcriptomic analysis.
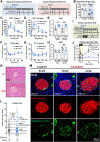
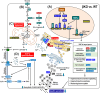
Results: Four findings are pinpointed. Firstly, β-cell specific Cisd2KO in mice disrupts systemic glucose homeostasis via impairing β-granules synthesis and insulin secretion; hypertrophy of the β-islets and the presence of a loss of identity that affects certain β-cells. Secondly, Cisd2 deficiency leads to impairment of glucose-induced extracellular Ca2+ influx, which compromises Ca2+-mediated insulin secretory signaling, causing mitochondrial dysfunction and, thereby impairing insulin secretion in the MIN6-Cisd2KO β-cells. Thirdly, transcriptomic analysis of β-islets reveals that Cisd2 modulates proteostasis and ER stress, mitochondrial function, insulin secretion and vesicle transport. Finally, the activated state of two potential upstream regulators, Glis3 and Hnf1a, is significantly suppressed under Cisd2 deficiency; notably, their downstream target genes are deeply involved in β-cell function and identity.
Conclusions: These findings provide mechanistic insights and form a basis for developing therapeutics for the effective treatment of diabetes in WFS2 patients.
Keywords: CISD2; Ca(2+) homeostasis; Diabetes; Mitochondrial function; Wolfram syndrome 2.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Loncke J., Vervliet T., Parys J.B., Kaasik A., Bultynck G. Uniting the divergent wolfram syndrome-linked proteins WFS1 and CISD2 as modulators. Sci Signal. 2021;14 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

