Double-negative T cells in combination with ursodeoxycholic acid ameliorates immune-mediated cholangitis in mice
- PMID: 40189495
- PMCID: PMC11974204
- DOI: 10.1186/s12916-025-04043-9
Double-negative T cells in combination with ursodeoxycholic acid ameliorates immune-mediated cholangitis in mice
Abstract
Background: Primary biliary cholangitis (PBC) is a liver-specific autoimmune disease. Treatment of PBC with ursodeoxycholic acid (UDCA) is not sufficient to prevent disease progression. Our previous study revealed that the number of hepatic double-negative T cells (DNT), which are unique regulatory T cells, was reduced in PBC patients. However, whether replenishment of DNT can prevent the progression of PBC remains unclear.
Methods: DnTGFβRII (Tg) mice and 2OA-BSA-immunized mice received DNT alone or in combination with oral UDCA. After 6-12 weeks of treatment, these mice were assessed for serological changes, liver pathological manifestations and intrahepatic immune responses.
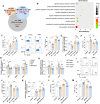
Results: Adoptive transfer of DNT alone significantly decreased serum levels of alanine transaminase (ALT), aspartate transaminase (AST), antimitochondrial antibody M2 (AMA-M2) and immunoglobulin M (IgM) in both Tg and 2OA-BSA-immunized PBC mouse models. In addition, DNT exhibited a strong killing effect on liver T cells and strong inhibition of their proliferation, but did not significantly improve the histology of PBC liver. However, combination therapy with DNT and oral UDCA predominantly ameliorated liver inflammation and significantly inhibited hepatic T and B cells. In vitro further study revealed that UDCA up-regulated the proliferation of DNT, increased the expression of the functional molecule perforin, and reduced the expression of NKG2A and endothelial cell protein C receptor (EPCR) through the farnesoid X receptor (FXR)/JNK signaling pathway in both mice and human DNT.
Conclusions: A single transfer of DNT ameliorated PBC in mice, while combination therapy of DNT with oral UDCA displayed a better efficacy, with stronger inhibition of hepatic T and B cells. This study highlights the potential application of DNT-based combination therapy for PBC, especially for UDCA non-responders.
Keywords: Double-negative T cells; Farnesoid X receptor; Primary biliary cholangitis; Ursodeoxycholic acid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The human DNT study was approved by the Research Ethics Committee of Beijing Friendship Hospital under ethics approval number 2020-P2-196-02, and informed consent from all healthy volunteers was obtained. All of the mice were housed and maintained in a pathogen-free, temperature-controlled environment at the Beijing Friendship Hospital under approval number 21-2011 for animal housing and use. All experimental procedures were conducted in accordance with the protocol approved by the Institutional Animal Care and Ethics Committee at Beijing Friendship Hospital and with the National Institutes of Health guidelines for the care and use of laboratory animals. Consent for publication: All authors read and approved the manuscript. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Decreased infiltration of CD4+ Th1 cells indicates a good response to ursodeoxycholic acid (UDCA) in primary biliary cholangitis.Pathol Res Pract. 2021 Jan;217:153291. doi: 10.1016/j.prp.2020.153291. Epub 2020 Nov 16. Pathol Res Pract. 2021. PMID: 33249399
-
Ursodeoxycholic acid impairs liver-infiltrating T-cell chemotaxis through IFN-γ and CX3CL1 production in primary biliary cholangitis.Eur J Immunol. 2021 Jun;51(6):1519-1530. doi: 10.1002/eji.202048589. Epub 2021 Apr 13. Eur J Immunol. 2021. PMID: 33710617
-
Evidence for the association between IgG-antimitochondrial antibody and biochemical response to ursodeoxycholic acid treatment in primary biliary cholangitis.J Gastroenterol Hepatol. 2017 Mar;32(3):659-666. doi: 10.1111/jgh.13534. J Gastroenterol Hepatol. 2017. PMID: 27529417
-
Obeticholic acid for the treatment of primary biliary cholangitis.Expert Opin Pharmacother. 2016 Sep;17(13):1809-15. doi: 10.1080/14656566.2016.1218471. Epub 2016 Aug 9. Expert Opin Pharmacother. 2016. PMID: 27468093 Review.
-
Primary Biliary Cholangitis: Medical and Specialty Pharmacy Management Update.J Manag Care Spec Pharm. 2016 Oct;22(10-a-s Suppl):S3-S15. doi: 10.18553/jmcp.2016.22.10-a-s.s3. J Manag Care Spec Pharm. 2016. PMID: 27700211 Free PMC article. Review.
References
-
- Mayo MJ. Mechanisms and molecules: What are the treatment targets for primary biliary cholangitis? Hepatology. 2022;76:518–31. - PubMed
-
- Melchor-Mendoza YK, Martinez-Benitez B, Mina-Hawat A, Rodriguez-Leal G, Duque X, Moran-Villota S. Ursodeoxycholic acid therapy in patients with primary biliary cholangitis with limited liver transplantation availability. Ann Hepatol. 2017;16:430–5. - PubMed
-
- Brandt D, Hedrich CM. TCRαβ(+)CD3(+)CD4(-)CD8(-) (double negative) T cells in autoimmunity. Autoimmun Rev. 2018;17:422–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

