The pharmacodynamics-based prophylactic benefits of GLP-1 receptor agonists and SGLT2 inhibitors on neurodegenerative diseases: evidence from a network meta-analysis
- PMID: 40189519
- PMCID: PMC11974209
- DOI: 10.1186/s12916-025-04018-w
The pharmacodynamics-based prophylactic benefits of GLP-1 receptor agonists and SGLT2 inhibitors on neurodegenerative diseases: evidence from a network meta-analysis
Abstract
Background: Glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors represent a new generation of antihyperglycemic agents that operate through mechanisms distinct from conventional diabetes treatments. Beyond their metabolic effects, these medications have demonstrated neuroprotective properties in preclinical studies. While clinical trials have explored their therapeutic potential in established neurodegenerative conditions, their role in disease prevention remains unclear. We conducted a network meta-analysis (NMA) to comprehensively evaluate the prophylactic benefits of these agents across multiple neurodegenerative diseases and identify the most promising preventive strategies.
Methods: We systematically searched PubMed, Embase, ClinicalKey, Cochrane CENTRAL, ProQuest, ScienceDirect, Web of Science, and ClinicalTrials.gov through October 24th, 2024, for randomized controlled trials (RCTs) of GLP-1 receptor agonists or SGLT2 inhibitors. Our primary outcome was the incidence of seven major neurodegenerative diseases: Parkinson's disease, Alzheimer's disease, Lewy body dementia, multiple sclerosis, amyotrophic lateral sclerosis, frontotemporal dementia, and Huntington's disease. Secondary outcomes included safety profiles assessed through dropout rates. We performed a frequentist-based NMA and evaluated risk of bias with Risk of Bias tool. The main result of the primary outcome in the current study would be re-affirmed via sensitivity test with Bayesian-based NMA.
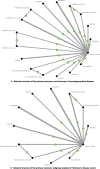
Results: Our analysis encompassed 22 RCTs involving 138,282 participants (mean age 64.8 years, 36.4% female). Among all investigated medications, only dapagliflozin demonstrated significant prophylactic benefits, specifically in preventing Parkinson's disease (odds ratio = 0.28, 95% confidence intervals = 0.09 to 0.93) compared to controls. Neither GLP-1 receptor agonists nor other SGLT2 inhibitors showed significant preventive effects for any of the investigated neurodegenerative conditions. Drop-out rates were comparable across all treatments.
Conclusions: This comprehensive NMA reveals a novel and specific prophylactic effect of dapagliflozin against Parkinson's disease, representing a potential breakthrough in preventive neurology. The specificity of dapagliflozin's protective effect to Parkinson's disease might rely on its highly selective inhibition to SGLT2. These findings provide important direction for future research and could inform preventive strategies for populations at risk of Parkinson's disease.
Trial registration: PROSPERO CRD42021252381.
Keywords: GLP-1 receptor agonist; Network meta-analysis; Neurodegenerative disease; Parkinson’s disease; SGLT2 inhibitor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The authors report no financial interests or potential conflicts of interest. The Institutional Review Board of the Tri-Service General Hospital has confirmed that no ethical approval is required (TSGHIRB: B-109–29). The current study did not directly involve individual participant so that we did not have the chance to approach individual participant or explore individual participant’s information. Therefore, it would be impossible to obtain consent to participate in the current study. The authors of this work were supported by the following grants: Brendon Stubbs is supported by the NIHR. Brendon Stubbs is part funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. Brendon Stubbs is also supported by the Maudsley Charity, King’s College London. Consent for publication: The current study did not directly involve individual participant so that we did not have the chance to approach individual participant or explore individual participant’s information. Therefore, it would be impossible to obtain consent to publish in the current study. Competing interests: The authors declare no competing interests.
Figures


References
-
- Avogaro A, de Kreutzenberg SV, Morieri ML, Fadini GP, Del Prato S. Glucose-lowering drugs with cardiovascular benefits as modifiers of critical elements of the human life history. Lancet Diabetes Endocrinol. 2022;10(12):882–9. - PubMed
-
- Erbil D, Eren CY, Demirel C, Kucuker MU, Solaroglu I, Eser HY. GLP-1’s role in neuroprotection: a systematic review. Brain Inj. 2019;33(6):734–819. - PubMed
-
- Plosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74(7):807–24. - PubMed
-
- Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

