Mechanistic insights into the structure-based design of a CspZ-targeting Lyme disease vaccine
- PMID: 40189575
- PMCID: PMC11973211
- DOI: 10.1038/s41467-025-58182-x
Mechanistic insights into the structure-based design of a CspZ-targeting Lyme disease vaccine
Abstract
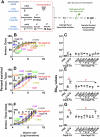
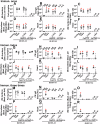
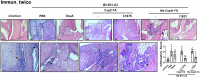
Borrelia burgdorferi (Bb) causes Lyme disease (LD), one of the most common vector-borne diseases in the Northern Hemisphere. Here, we solve the crystal structure of a mutated Bb vaccine antigen, CspZ-YA that lacks the ability to bind to host complement factor H (FH). We generate point mutants of CspZ-YA and identify CspZ-YAI183Y and CspZ-YAC187S to trigger more robust bactericidal responses. Compared to CspZ-YA, these CspZ-YA mutants require a lower immunization frequency to protect mice from LD-associated inflammation and bacterial colonization. Antigenicity of wild-type and mutant CspZ-YA proteins are similar, as measured using sera from infected people or immunized female mice. Structural comparison of CspZ-YA with CspZ-YAI183Y and CspZ-YAC187S shows enhanced interactions of two helices adjacent to the FH-binding sites in the mutants, consistent with their elevated thermostability. In line with these findings, protective CspZ-YA monoclonal antibodies show increased binding to CspZ-YA at a physiological temperature (37 °C). In summary, this proof-of-concept study applies structural vaccinology to enhance intramolecular interactions for the long-term stability of a Bb antigen while maintaining its protective epitopes, thus promoting LD vaccine development.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.L. is the inventor of US patent application no. US11771750B2 (“Composition and method for generating immunity to Borrelia burgdorferi”). The remaining authors declare no competing interests.
Figures



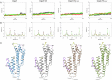
Update of
-
Mechanistic insights into structure-based design of a Lyme disease subunit vaccine.bioRxiv [Preprint]. 2024 Oct 28:2024.10.23.619738. doi: 10.1101/2024.10.23.619738. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 07;16(1):2898. doi: 10.1038/s41467-025-58182-x. PMID: 39554036 Free PMC article. Updated. Preprint.
References
-
- Principi, N. & Esposito, S. Adverse events following immunization: real causality and myths. Expert Opin. Drug Saf.15, 825–835 (2016). - PubMed
-
- Kimmel, S. R., Burns, I. T., Wolfe, R. M. & Zimmerman, R. K. Addressing immunization barriers, benefits, and risks. J. Fam. Pr.56, S61–S69 (2007). - PubMed
-
- Schellekens, H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat. Rev. Drug Discov.1, 457–462 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- R21 AI144891/AI/NIAID NIH HHS/United States
- R01AI154542/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R44 AI152954/AI/NIAID NIH HHS/United States
- R21AI144891/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- W81XWH-20-1-0913/United States Department of Defense | United States Army | Army Medical Command | Congressionally Directed Medical Research Programs (CDMRP)
- R01AI181746/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI181746/AI/NIAID NIH HHS/United States
- R44AI152954/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI154542/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

