Folliculin depletion results in liver cell damage and cholangiocarcinoma through MiT/TFE activation
- PMID: 40189703
- PMCID: PMC12325662
- DOI: 10.1038/s41418-025-01486-8
Folliculin depletion results in liver cell damage and cholangiocarcinoma through MiT/TFE activation
Abstract
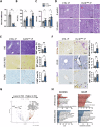
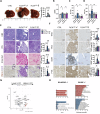
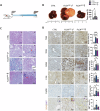
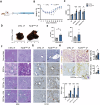
Mutations in the tumor suppressor gene Folliculin (FLCN) are responsible for Birt-Hogg-Dube' (BHD) syndrome, a rare inherited condition that predisposes affected individuals to skin tumors, pulmonary cysts, and kidney tumors. FLCN regulates key cellular pathways, including TFEB, TFE3, and mTORC1, which are critical for maintaining cell homeostasis. Loss of FLCN leads to both hyperactivation of mTORC1 and constitutive activation of TFEB and TFE3, contributing to tumorigenesis. While previous studies showed that Flcn liver-specific conditional knockout (FlcnLiKO) mice are protected from developing liver fibrosis and damage upon high-fat diet exposure, the potential role of FLCN loss in liver carcinogenesis remained unexplored. Here, we demonstrate that hepatic loss of FLCN in mice results in cancer associated with inflammation and fibrosis with features of cholangiocarcinoma (CCA). This phenotype emerges in mice over 90-week-old, with a male predominance. Moreover, FlcnLiKO mice are more prone to develop diethylnitrosamine (DEN)- or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)- induced liver tumors with heterogenous histological features. Notably, depletion of TFE3, but not TFEB, in the liver of FlcnLiKO mice fully rescues the cancer phenotype and normalized mTORC1 signaling, highlighting TFE3 as the primary driver of liver cancer and mTORC1 hyperactivity in the absence of FLCN.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: AB is cofounder and shareholder of CASMA Therapeutics, Inc, and Advisory board member of Avilar Therapeutics and Amplify Therapeutics. Ethics approval: The manuscript reporting studies did not involve human participants, human data or human tissue. The animal experiments were accomplished in compliance with ethical standards. All experiments were approved by the Italian Ministry of Health with the approved number CE571.65 and were performed in accordance with the legal mandates and federal guidelines for the care and maintenance of laboratory animals.
Figures

References
MeSH terms
Substances
Grants and funding
- NA/Fondazione Telethon (Telethon Foundation)
- MFAG-24872/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- IG-22103/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- AdG; INCANTAR/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Excellent Science (H2020 Priority Excellent Science)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

