Rosuvastatin-based combination treatment with acetylsalicylic acid or ezetimibe in the management of patients at high and very high cardiovascular risk. Expert opinion paper of the Polish Lipid Association 2025
- PMID: 40190297
- PMCID: PMC11969506
- DOI: 10.5114/aoms/199826
Rosuvastatin-based combination treatment with acetylsalicylic acid or ezetimibe in the management of patients at high and very high cardiovascular risk. Expert opinion paper of the Polish Lipid Association 2025
Abstract
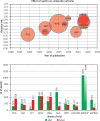
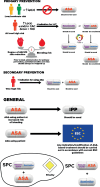
Lipid disorders are the most common risk factor for atherosclerotic cardiovascular disease (ASCVD) in Poland, where it is responsible for up to 200,000 deaths per year, with the number of myocardial infarctions and strokes reaching 80,000 annually and 25% of people dying within 3 years after a myocardial infarction. Despite the availability of effective drugs, the level of control of low-density lipoprotein cholesterol (LDL-C) is low, at only about 20% among high- and very high-risk patients, who often require combination lipid-lowering therapy (LLT) with a potent statin (e.g. rosuvastatin) and ezetimibe. Moreover, in Poland, several million patients require concomitant lipid-lowering and antiplatelet (acetylsalicylic acid) therapy based on their risks and indications. Single pill combinations (SPCs) improve adherence to treatment as well as the achievement of therapeutic goals and allow a greater reduction in cardiovascular incidents and mortality. This expert opinion paper, endorsed by the Polish Lipid Association (PoLA), provides practical recommendations for more effective treatment of patients with indications for LLT and antiplatelet therapy using available rosuvastatin-based combination therapies (with ezetimibe or acetylsalicylic acid).
Keywords: cardiovascular risk; combination therapies; ezetimibe; acetylsalicylic acid; primary and secondary prevention; rosuvastatin.
Copyright: © 2025 Termedia & Banach.
Conflict of interest statement
Maciej Banach has received grant(s)/research support from Amgen, Daiichi-Sankyo, Mylan/Viatris, and Sanofi and has served as a consultant and/or received speaker’s fees from Adamed, Amgen, Exceed Pharma, Daiichi-Sankyo, Esperion, Kogen, KRKA, Lilly, MSD, Mylan/Viatris, NewAmsterdam Pharma, Novartis, Novo-Nordisk, Polpharma, Sanofi, Teva and Zentiva; Stanislaw Surma has received fees from Novartis/Sandoz, KRKA and Pro.Med; Marek Gierlotka: speaker and/or consulting fees from Adamed, Amgen, Astra-Zeneca, Bayer, Berlin-Chemie Menarini, Boehringer Ingelheim, Merck, Novartis, Novo Nordisk, Promed, Sandoz, Sanofi; Piotr Jankowski has received grant(s)/research support and speaker’s fees from Adamed, Amgen, KRKA, Novartis, Novo-Nordisk, Polpharma, Sanofi, and Zentiva; Jacek Józwiak: has received speaker and/or consulting fees from Valeant, BIO-RAD, Euroimmun, Adamed, Amgen, Boehringer Ingelheim, Teva, Zentiva, Celgene, Servier, Bioton and ALAB; Jacek Kubica: has received speaker and/or consulting fees from Adamed, AstraZeneca, Boehringer Ingelheim, Ferrer, Gedeon Richter and Servier; Tomasz Tomasik has received fees for consulting and educational services from: Biofarm, Adamed, Boehringer, Astra Zeneca, Novo-Nordisk and Teva; Robert Gil: speaker and/or consulting fees from: Amgen, Novartis, AstraZeneca, Boehringer Ingelheim, Gedeon Richter, Pfizer, Servier; all other authors do not declare any conflict of interest.
Figures

References
-
- Roth GA, Mensah GA, Johnson CO, et al. .; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020; 76: 2982–3021. Erratum in: J Am Coll Cardiol 2021; 77: 1958-9. - PMC - PubMed
-
- Khalid N, Abdullah M, Afzal MA, et al. . A systematic analysis of Global Disease Burden of ischemic heart disease from 1990-2019. J Am Coll Cardiol 2024, 83 (13 Suppl): 1338. - PubMed
LinkOut - more resources
Full Text Sources
