Adaptive Whole-Brain Dynamics Predictive Method: Relevancy to Mental Disorders
- PMID: 40190349
- PMCID: PMC11971527
- DOI: 10.34133/research.0648
Adaptive Whole-Brain Dynamics Predictive Method: Relevancy to Mental Disorders
Abstract
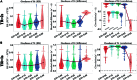
The Hopf whole-brain model, based on structural connectivity, overcomes limitations of traditional structural or functional connectivity-focused methods by incorporating heterogeneity parameters, quantifying dynamic brain characteristics in healthy and diseased states. Traditional parameter fitting techniques lack precision, restricting broader use. To address this, we validated parameter fitting methods using simulated networks and synthetic models, introducing improvements such as individual-specific initialization and optimized gradient descent, which reduced individual data loss. We also developed an approximate loss function and gradient adjustment mechanism, enhancing parameter fitting accuracy and stability. Applying this refined method to datasets for major depressive disorder (MDD) and autism spectrum disorder (ASD), we identified differences in brain regions between patients and healthy controls, explaining related anomalies. This rigorous validation is crucial for clinical application, paving the way for precise neuropathological identification and novel treatments in neuropsychiatric research, demonstrating substantial potential in clinical neurology.
Copyright © 2025 Qian-Yun Zhang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Horwitz B, Tagamets MA, McIntosh AR. Neural modeling, functional brain imaging, and cognition. Trends Cogn Sci. 1999;3(3):91–98. - PubMed
-
- Jirsa VK, Jantzen KJ, Fuchs A, Kelso JS. Spatiotemporal forward solution of the EEG and MEG using network modeling. IEEE Trans Med Imaging. 2002;21(5):493–504. - PubMed
LinkOut - more resources
Full Text Sources

