Enduring differential patterns of neuronal loss and myelination along 6-month pulsatile gonadotropin-releasing hormone therapy in individuals with Down syndrome
- PMID: 40190351
- PMCID: PMC11969670
- DOI: 10.1093/braincomms/fcaf117
Enduring differential patterns of neuronal loss and myelination along 6-month pulsatile gonadotropin-releasing hormone therapy in individuals with Down syndrome
Abstract
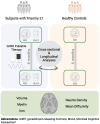
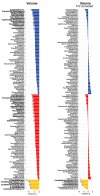
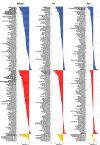
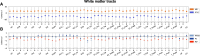
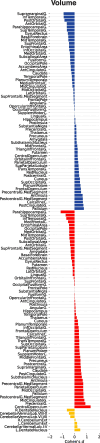
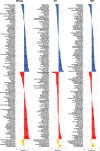
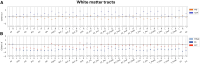
Despite major progress in understanding the impact of the triplicated chromosome 21 on the brain and behaviour in Down syndrome, our knowledge of the underlying neurobiology in humans is still limited. We sought to address some of the pertinent questions about the drivers of brain structure differences and their associations with cognitive function in Down syndrome. To this aim, in a pilot magnetic resonance imaging (MRI) study, we monitored brain anatomy in individuals with Down syndrome receiving pulsatile gonadotropin-releasing hormone (GnRH) therapy over 6 months in comparison with typically developed age- and sex-matched healthy controls. We analysed cross-sectional (Down syndrome/healthy controls n = 11/27; Down syndrome-2 females/9 males, age 26.7 ± 5.0 years old; healthy controls-8 females/19 males, age 24.1 ± 2.5 years old) and longitudinal (Down syndrome/healthy controls n = 8/13; Down syndrome-1 female/7 males, age 26.4 ± 5.3 years old; healthy controls-4 females/9 males, 24.7 ± 2.2 years old) relaxometry and diffusion-weighted MRI data alongside standard cognitive assessment. The statistical tests looked for cross-sectional baseline differences and for differential changes over time between Down syndrome and healthy controls. The post hoc analysis confined to the Down syndrome group, tested for potential time-dependent interactions between individuals' overall cognitive performance and associated brain anatomy changes. The brain MRI statistical analyses covered both grey and white matter regions across the whole brain allowing for investigation of regional volume, macromolecular/myelin and iron content, additionally to diffusion tensor and neurite orientation and dispersion density characterization across major white matter tracts. The cross-sectional analysis showed reduced frontal, temporal and cerebellar volumes in Down syndrome with only the cerebellar differences remaining significant after adjustment for the presence of microcephaly (P family-wise-corrected < 0.05). The volume reductions were paralleled by decreased cortical and subcortical macromolecular/myelin content confined to the cortical motor system, thalamus and basal ganglia (P family-wise-corrected < 0.05). All major white matter tracts showed a ubiquitous mean diffusivity and intracellular volume fraction reduction contrasted with no differences in magnetization transfer saturation metrics (P family-wise-corrected < 0.05). Compared with healthy controls over the same period, Down syndrome individuals under GnRH therapy showed cognitive improvement (Montreal Cognitive Assessment from 11.4 ± 5.5 to 15.1 ± 5.6; P < 0.01) on the background of stability of the observed differential neuroanatomical patterns. Despite the lack of adequate Down syndrome control group, we interpret the obtained cross-sectional and longitudinal findings in young adults as evidence for predominant neurodevelopmental neuronal loss due to dysfunctional neurogenesis without signs for short-term myelin loss.
Keywords: brain tissue properties; magnetic resonance imaging; morphometry; multi-parameter mapping; trisomy 21.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Schmidt-Sidor B, Wisniewski KE, Shepard TH, Sersen EA. Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin Neuropathol. 1990;9(4):181–190. - PubMed
-
- Wisniewski KE. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Genet Suppl. 1990;7:274–281. - PubMed
-
- Stagni F, Giacomini A, Emili M, Guidi S, Bartesaghi R. Neurogenesis impairment: An early developmental defect in Down syndrome. Free Radic Biol Med. 2018;114:15–32. - PubMed
LinkOut - more resources
Full Text Sources
