Clinical efficacy of etonogestrel implants on relieving dysmenorrhea in endometriosis and adenomyosis women for up to 3 years
- PMID: 40190575
- PMCID: PMC11968362
- DOI: 10.3389/fmed.2025.1460578
Clinical efficacy of etonogestrel implants on relieving dysmenorrhea in endometriosis and adenomyosis women for up to 3 years
Abstract
Background: Dysmenorrhea and menstrual disorders caused by endometriosis (EM) and adenomyosis (AM) have significantly affected the quality of life of a large number of women. As a highly effective clinical contraceptive measure, etonogestrel implants have been previously reported to relieve dysmenorrhea. However, the dysmenorrhea treatment and menstrual regulation effects of etonogestrel implants in AM and EM patients have not been systematically studied.
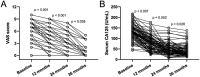
Methods: This retrospective study followed up 100 patients with etonogestrel implants from May 2015 to October 2016, including 44 patients with EM and 56 patients with AM. The VAS scores of dysmenorrhea, menstrual volume, and related adverse events were measured at 12, 24, and 36 months after etonogestrel implantation in these patients.
Results: In 100 EM and AM patients, dysmenorrhea significantly improved, with moderate and severe cases decreasing from 50 to 16 and 0% at 36 months. Amenorrhea increased over time, and frequent bleeding declined. Adverse reactions included weight gain (21%), acne (13%), and decreased sexual desire (10%). Serum CA125 levels dropped, confirming therapeutic efficacy.
Conclusion: Etonogestrel implantation significantly alleviated dysmenorrhea symptoms in AM and EM patients.
Keywords: adenomyosis; dysmenorrhea; endometriosis; etonogestrel implants; women.
Copyright © 2025 Li, Li, Feng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A clinical observational study on the efficacy of subcutaneous etonogestrel implants for adenomyosis in 20 patients.Gynecol Endocrinol. 2021 Aug;37(8):735-739. doi: 10.1080/09513590.2021.1922886. Epub 2021 Jun 23. Gynecol Endocrinol. 2021. PMID: 34160336
-
Treatment of Adenomyosis with Subcutaneous Etonogestrel Implants: A Clinical Observational Study in 17 Patients.Med Sci Monit. 2018 Sep 1;24:6085-6092. doi: 10.12659/MSM.908979. Med Sci Monit. 2018. PMID: 30171680 Free PMC article.
-
Effects of Etonogestrel implants on pelvic pain and menstrual flow in women suffering from adenomyosis or endometriosis: Results from a prospective, observational study.Medicine (Baltimore). 2021 Feb 12;100(6):e24597. doi: 10.1097/MD.0000000000024597. Medicine (Baltimore). 2021. PMID: 33578561 Free PMC article.
-
The use of progestin subdermal implants in the management of endometriosis-related pain symptoms and quality of life: a systematic review.Curr Med Res Opin. 2022 Mar;38(3):479-486. doi: 10.1080/03007995.2022.2031144. Epub 2022 Feb 1. Curr Med Res Opin. 2022. PMID: 35048754
-
Menstruation and its disorders in adolescence.Curr Probl Pediatr. 1982 Aug;12(10):1-61. doi: 10.1016/0045-9380(82)90034-2. Curr Probl Pediatr. 1982. PMID: 6764754 Review.
Cited by
-
The Sebaceous Gland: A Key Player in the Balance Between Homeostasis and Inflammatory Skin Diseases.Cells. 2025 May 20;14(10):747. doi: 10.3390/cells14100747. Cells. 2025. PMID: 40422250 Free PMC article. Review.
References
-
- Shi J, Dai Y, Zhang J, Li X, Jia S, Leng J. Pregnancy outcomes in women with infertility and coexisting endometriosis and adenomyosis after laparoscopic surgery: a long-term retrospective follow-up study. BMC Pregnancy Childbirth. (2021) 21:383. doi: 10.1186/s12884-021-03851-0, PMID: - DOI - PMC - PubMed
-
- Deng S, Lang JH, Leng JH, Liu ZF, Sun DW, Zhu L. Effects of levonorgestrel-releasing intrauterine system on pain and recurrence associated with endometriosis and adenomyosis. Zhonghua Fu Chan Ke Za Zhi. (2006) 41:664–8. PMID: - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

