The pulsing brain: state of the art and an interdisciplinary perspective
- PMID: 40191028
- PMCID: PMC11969196
- DOI: 10.1098/rsfs.2024.0058
The pulsing brain: state of the art and an interdisciplinary perspective
Abstract
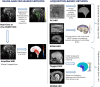
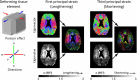
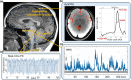
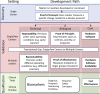
Understanding the pulsing dynamics of tissue and fluids in the intracranial environment is an evolving research theme aimed at gaining new insights into brain physiology and disease progression. This article provides an overview of related research in magnetic resonance imaging, ultrasound medical diagnostics and mathematical modelling of biological tissues and fluids. It highlights recent developments, illustrates current research goals and emphasizes the importance of collaboration between these fields.
Keywords: brain tissue pulsation; magnetic resonance imaging medical diagnostics; mathematical modelling of biological fluids; mathematical modelling of biological tissues; ultrasound medical diagnostics.
© 2025 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures
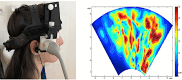
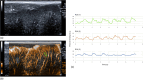
References
-
- Fishman AP, Richards DW. 1982. The cerebral circulation. In Circulation of the blood (eds Fishman AP, Richards DW), pp. 703–742. New York, NY: Springer. ( 10.1007/978-1-4614-7546-0_11) - DOI
Publication types
LinkOut - more resources
Full Text Sources
