Adjuvanticity of Tannic Acid-Modified Nanoparticles Improves Effectiveness of the Antiviral Response
- PMID: 40191045
- PMCID: PMC11972000
- DOI: 10.2147/IJN.S512509
Adjuvanticity of Tannic Acid-Modified Nanoparticles Improves Effectiveness of the Antiviral Response
Abstract
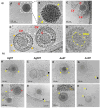
Introduction: Herpes simplex virus type 1 (HSV-1) causes recurrent infections of skin and mucosal tissues with high global prevalence. HSV-1 also invades the nervous system where it establishes a lifelong latency-making infection poorly treatable We previously showed that both tannic acid-modified silver and gold nanoparticles (TA-Ag/AuNPs) inhibit HSV-1 infection in vitro.
Methods: We used an in vitro and in vivo model of HSV-1 infection to study how metal type, size and tannic acid modification of nanoparticles can influence development of the early innate response and the mounting of specific anti-HSV-1 response upon treatment of the nasal mucosa.
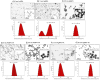
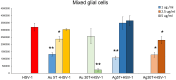
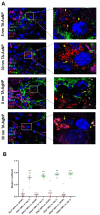
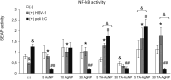
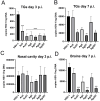
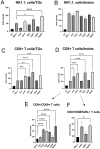
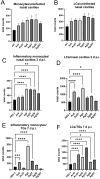
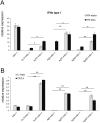
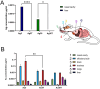
Results: We found that tannic acid is necessary for binding with HSV-1, with smaller sizes independent of the NPs composition, whereas for larger NPs, only TA-AgNPs can inhibit HSV-1 infection. Intranasal treatment of HSV-1 infection with TA-Ag/AuNPs results in lower viral titers and a better antiviral response, followed by increased IFN-α, CXCL9, and CXCL10 levels as well as infiltration of T cells and NK cells in the infected sites. We also found that the application of TA-NPs to the nasal cavities of infected mice induced infiltration of both monocytes and Langerhans cells (LCs), which lasted longer compared to the application of unmodified NPs. Furthermore, TA-NPs activated monocytes and microglia to produce antiviral cytokines and chemokines better than unmodified NPs, except for the large TA-AuNPs.
Discussion: Treatment of the mucosal tissues at the early stage of HSV-1 infection helps to modulate specific and effective antiviral immune response by attracting cytotoxic lymphocytes and inducing the production of antiviral cytokines and chemokines. Furthermore, tannic acid modification is helpful for the removal of nanoparticles from the respiratory tract, which increases the safety of nanoparticle applications to treat infections.
Keywords: AgNPs; AuNPs; HSV-1; microglia; tannic acid.
© 2025 Janicka et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Rodrigues I, Campo KN, Arns CW, Gabriel LP, Webster TJ, Lopes ÉSN. From bulk to nanoparticles: an overview of antiviral materials, its mechanisms, and applications. Part Part Syst Charact. 2021;38(8):2100044. doi: 10.1002/ppsc.202100044 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

