Clinical implementation of interstitial brachytherapy in early-stage oral cancer in a newly commissioned tertiary cancer center: Challenges and initial experience
- PMID: 40191055
- PMCID: PMC11966224
- DOI: 10.5114/jcb.2025.148374
Clinical implementation of interstitial brachytherapy in early-stage oral cancer in a newly commissioned tertiary cancer center: Challenges and initial experience
Abstract
Purpose: High-dose-rate (HDR) interstitial brachytherapy (ISBT) is a curative treatment option for head and neck cancer patients. However, its overall utilization has been declining, particularly in newer cancer setups. This study investigated challenges in ISBT implementation, and reported initial outcomes of early-stage oral cancer patients in a newly established tertiary cancer center.
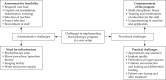
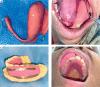
Material and methods: After reviewing guidelines and addressing administrative requirements, ISBT program was launched. Key steps in the process included establishing brachytherapy suite, staff training, and optimizing workflows. Alongside standard protocols, additional procedures, such as clinical drawing templates, intra-oral ultrasound, and intra-oral spacers were implemented. From August 2020 to July 2022, 18 patients with early-stage (cT1-2N0M0) oral cancer (tongue = 13, lip = 3, buccal mucosa = 2) received treatment with either ISBT alone (n = 3) or external beam radiotherapy (EBRT), followed by ISBT with HDR cobalt-60 source (n = 15). Treatment characteristics, oncological outcomes, and morbidity profiles were analyzed.
Results: The median age of the cohort was 55 years (range, 29-75 years), with two-thirds of males. The majority had T1 stage (72.2%), with infiltrative growth pattern (72.2%). All patients with oral tongue cancer, 1 lip and 1 buccal mucosa cancer, received elective nodal irradiation with EBRT, followed by ISBT, achieving a total median EQD2 of 74 Gy. The remaining 3 patients (2 with lip and 1 with buccal mucosa primary) received ISBT alone. Post-treatment complete response was observed in 17 patients (94.4%), with no cases of acute toxicity > grade 2. At a median follow-up of 32 months, an overall 3-year local-regional control and overall survival rates were 67.9% and 72.7%, respectively. One patient developed grade 3 myelopathy, and one grade 3 osteoradionecrosis.
Conclusions: Implementing ISBT in a newly established cancer center is feasible and effective for early-stage oral cancer, providing moderate oncological outcomes with manageable toxicity profile.
Keywords: head and neck cancer; interstitial brachytherapy; oral cancer.
Copyright © 2025 Termedia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Report of the Hospital Based Cancer Registries 2021. Accessed: Jul. 18, 2023 [Online]. Available: https://ncdirindia.org/All_Reports/HBCR_2021/
-
- Aupérin A. Epidemiology of head and neck cancers: An update. Curr Opin Oncol 2020; 32: 178-186. - PubMed
-
- Rudoltz MS, Perkins RS, Luthmann RWet al. . High-dose-rate brachytherapy for primary carcinomas of the oral cavity and oropharynx. Laryngoscope 1999; 109: 1967-1973. - PubMed
LinkOut - more resources
Full Text Sources
