Enhanced and long-lasting SARS-CoV-2 immune memory in individuals with common cold coronavirus cross-reactive T cell immunity
- PMID: 40191213
- PMCID: PMC11968687
- DOI: 10.3389/fimmu.2025.1501704
Enhanced and long-lasting SARS-CoV-2 immune memory in individuals with common cold coronavirus cross-reactive T cell immunity
Abstract
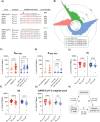
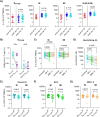
With the continuous emergence of novel SARS-CoV-2 variants, long-lasting and broadly reactive cellular and humoral immunity is critical for durable protection from COVID-19. We investigated SARS-CoV-2-specific T cell immunity in relation to antibodies, infection outcome and disease severity and assessed its durability in a longitudinal cohort over a three-year time course. We identified pre-existing T cells reactive to the seasonal coronavirus (CoV) OC43 that cross-react with the conserved SARS-CoV-2 spike S813-829 peptide. These cross-reactive T cells increased in frequency following SARS-CoV-2 infection or vaccination and correlated with enhanced spike-specific T cell responses and significantly reduced viral loads. Furthermore, our data revealed that CoV-cross-reactive T cells were maintained as part of the long-lasting memory response, contributing to increased T cell frequencies against omicron variants. These findings suggest a functional role of CoV-cross-reactive T cells that extends beyond the initial SARS-CoV-2 exposure, contributing to enhanced immunity against highly mutated SARS-CoV-2 variants.
Keywords: CD4 T cell response; SARS-CoV-2; cell-mediated immunity; long term immunity; mRNA vaccination.
Copyright © 2025 Florian, Bauer, Popovitsch, Fae, Springer, Graninger, Traugott, Weseslindtner, Aberle, Fischer, Kundi, Stiasny, Zoufaly, Landry and Aberle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

