Instantaneous Hydrolysis of Methyl Paraoxon Nerve Agent Simulant Is Catalyzed by Nontoxic Aminoguanidine Imines
- PMID: 40191310
- PMCID: PMC11966261
- DOI: 10.1021/acsomega.4c09946
Instantaneous Hydrolysis of Methyl Paraoxon Nerve Agent Simulant Is Catalyzed by Nontoxic Aminoguanidine Imines
Abstract
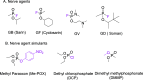
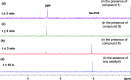
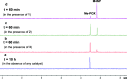
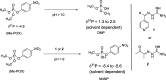
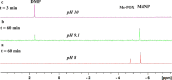
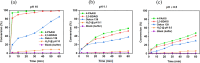
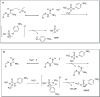
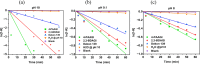
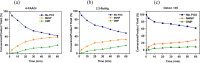
Exposure to organophosphate-based nerve agents and pesticides poses health and security threats to civilians, soldiers, and first responders. Thus, there is a need to develop effective decontamination agents that are nonhazardous to human health. To address this, we demonstrate that instantaneous hydrolysis of methyl paraoxon (Me-POX), a nerve agent simulant, can be achieved in the presence of aminoguanidine imines at pH 10: ● the pyridine-4-aldehyde aminoguanidine-imine (1) and ● the 2,3-butanedione aminoguanidine-imine (2). The hydrolysis of Me-POX under these conditions is substantially faster than that of the state-of-the-art decontaminating agent, Dekon-139 (2,3-butanedione oxime, potassium salt). Furthermore, Dekon-139 shows adverse effects when applied on skin surfaces, making it of great interest to develop safer but effective decontaminating agents for neutralizing nerve agents and pesticides exposed to skin-surface areas. Our pharmaceutically relevant aminoguanidine derivatives serve as rather nontoxic and safe decontaminating agents for organophosphate-based nerve agents and pesticides. The hydrolytic degradation products of Me-POX by our aminoguanidine-based imines and Dekon-139 are pH dependent. At pH > 10, Me-POX is hydrolyzed to give dimethyl phosphate as the exclusive product, whereas at pH < 9, the major product of hydrolysis is methyl 4-nitrophenyl phosphate (M4NP). We applied Quantum Mechanics calculations to investigate the mechanism of this dramatically accelerated decontamination process. We predict that in the rate-determining transition state, both 1 and 2 stabilize the reaction center through hydrogen bonding. Compared to Dekon-139, the rate constants of the rate-determine steps (RDS) are predicted to be over 9,000 times larger for 1 and over 600 times larger for 2, explaining the improvement. Quantum Mechanics calculations rationalize the pH-dependent hydrolysis products of the Me-POX in the gas phase, and gauge-including atomic orbital (GIAO)-31P NMR chemical shift calculations confirm the experimental values.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Hydrophilic scaffolds of oxime as the potent catalytic inactivator of reactive organophosphate.Chem Biol Interact. 2019 Jan 5;297:67-79. doi: 10.1016/j.cbi.2018.10.022. Epub 2018 Oct 25. Chem Biol Interact. 2019. PMID: 30393113
-
Product Inhibition and the Catalytic Destruction of a Nerve Agent Simulant by Zirconium-Based Metal-Organic Frameworks.ACS Appl Mater Interfaces. 2021 Jul 7;13(26):30565-30575. doi: 10.1021/acsami.1c05062. Epub 2021 Jun 23. ACS Appl Mater Interfaces. 2021. PMID: 34161064
-
In vitro skin decontamination of the organophosphorus pesticide Paraoxon with nanometric cerium oxide CeO2.Chem Biol Interact. 2017 Apr 1;267:57-66. doi: 10.1016/j.cbi.2016.04.035. Epub 2016 Apr 26. Chem Biol Interact. 2017. PMID: 27129420
-
Comparative efficacy of Reactive Skin Decontamination Lotion (RSDL): A systematic review.Toxicol Lett. 2021 Oct 1;349:109-114. doi: 10.1016/j.toxlet.2021.06.010. Epub 2021 Jun 17. Toxicol Lett. 2021. PMID: 34147606
-
Abiotic hydrolysis of pesticides in the aquatic environment.Rev Environ Contam Toxicol. 2002;175:79-261. Rev Environ Contam Toxicol. 2002. PMID: 12206055 Review.
Cited by
-
Efficient Decontamination of Organophosphate-Based Pesticides and Nerve Agent Simulants Mediated by N‑Containing Nucleophiles.ACS Omega. 2025 Aug 5;10(32):35878-35891. doi: 10.1021/acsomega.5c02810. eCollection 2025 Aug 19. ACS Omega. 2025. PMID: 40852278 Free PMC article.
References
-
- McGuire J. R.; Bester S. M.; Guelta M. A.; Cheung J.; Langley C.; Winemiller M. D.; Bae S. Y.; Funk V.; Myslinski J. M.; Pegan S. D.; Height J. J. Structural and Biochemical Insights into the Inhibition of Human Acetylcholinesterase by G-Series Nerve Agents and Subsequent Reactivation by HI-6. Chem. Res. Toxicol. 2021, 34, 804–816. 10.1021/acs.chemrestox.0c00406. - DOI - PubMed
LinkOut - more resources
Full Text Sources
